

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333767 (CHEMBL1644288 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.59 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333763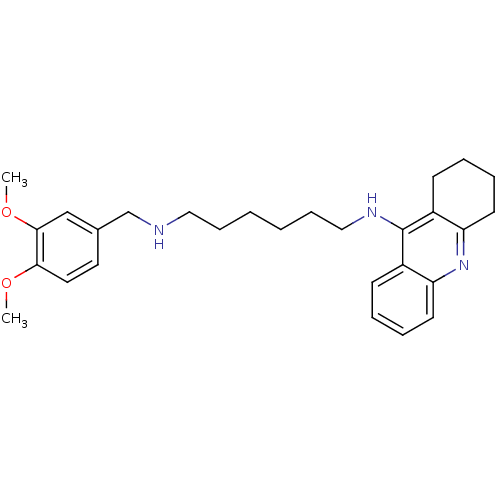 (CHEMBL1644292 | N1-(3,4-Dimethoxybenzyl)-N6-(1,2,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.68 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333765 (CHEMBL1644290 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333766 (CHEMBL1644289 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.55 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333764 (CHEMBL1644291 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.73 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333771 (CHEMBL1644284 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333762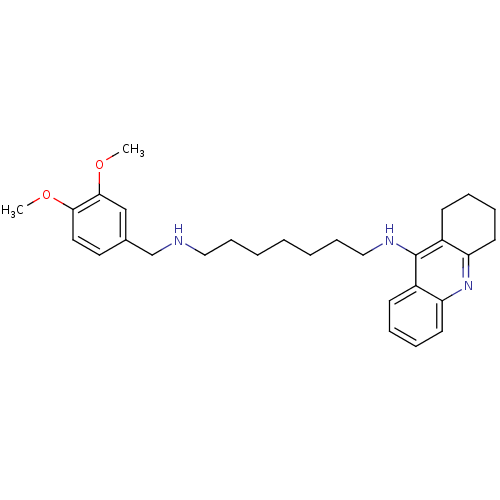 (CHEMBL1644293 | N1-(3,4-Dimethoxybenzyl)-N7-(1,2,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333761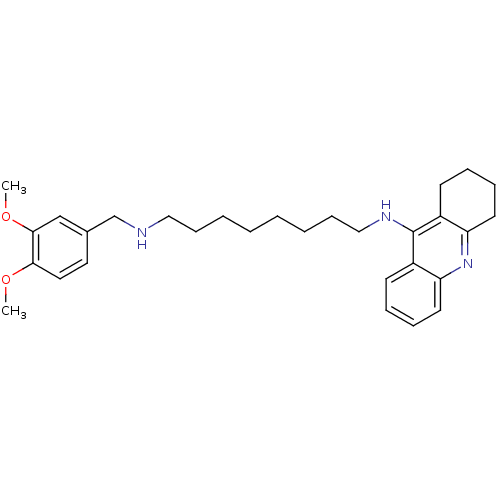 (CHEMBL1644294 | N1-(3,4-Dimethoxybenzyl)-N8-(1,2,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.57 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333760 (CHEMBL1644295 | N1-(3,4-Dimethoxybenzyl)-N9-(1,2,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.67 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333769 (CHEMBL1644286 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333769 (CHEMBL1644286 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.98 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333770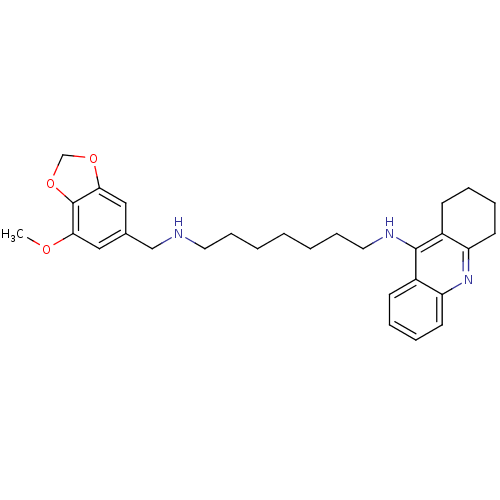 (CHEMBL1644285 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.73 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333765 (CHEMBL1644290 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.77 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333761 (CHEMBL1644294 | N1-(3,4-Dimethoxybenzyl)-N8-(1,2,3...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333768 (CHEMBL1644287 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333766 (CHEMBL1644289 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333770 (CHEMBL1644285 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333768 (CHEMBL1644287 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333764 (CHEMBL1644291 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333771 (CHEMBL1644284 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333767 (CHEMBL1644288 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333762 (CHEMBL1644293 | N1-(3,4-Dimethoxybenzyl)-N7-(1,2,3...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24.0 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333760 (CHEMBL1644295 | N1-(3,4-Dimethoxybenzyl)-N9-(1,2,3...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 27.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333772 (CHEMBL1644283 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333763 (CHEMBL1644292 | N1-(3,4-Dimethoxybenzyl)-N6-(1,2,3...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333773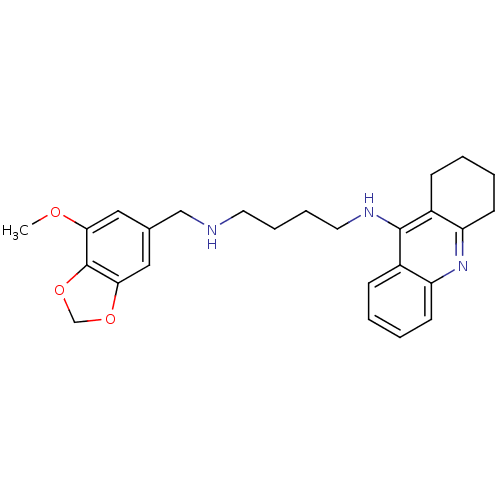 (CHEMBL1644282 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333772 (CHEMBL1644283 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 78.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333773 (CHEMBL1644282 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 193 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||