 Found 68 hits of Enzyme Inhibition Constant Data
Found 68 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50386178
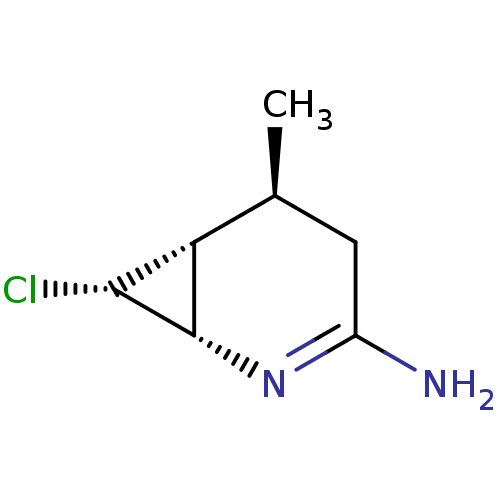
(CHEMBL1800346 | ONO-1714)Show SMILES C[C@H]1CC(N)=N[C@@H]2[C@H](Cl)[C@H]12 |r,c:4| Show InChI InChI=1S/C7H11ClN2/c1-3-2-4(9)10-7-5(3)6(7)8/h3,5-7H,2H2,1H3,(H2,9,10)/t3-,5?,6+,7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50386178

(CHEMBL1800346 | ONO-1714)Show SMILES C[C@H]1CC(N)=N[C@@H]2[C@H](Cl)[C@H]12 |r,c:4| Show InChI InChI=1S/C7H11ClN2/c1-3-2-4(9)10-7-5(3)6(7)8/h3,5-7H,2H2,1H3,(H2,9,10)/t3-,5?,6+,7-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124535
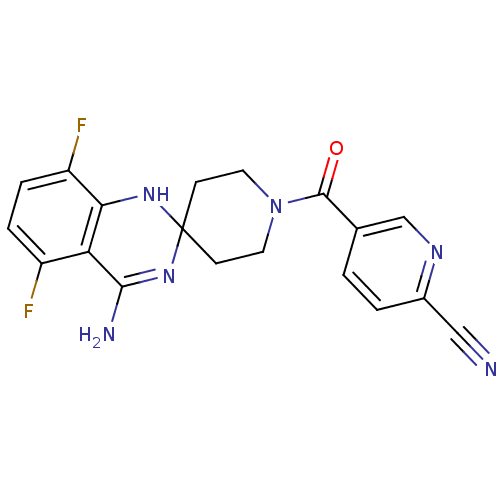
(1-(6-CYANO-3-PYRIDYLCARBONYL)-5',8'-DIFLUOROSPIRO[...)Show SMILES NC1=NC2(CCN(CC2)C(=O)c2ccc(nc2)C#N)Nc2c(F)ccc(F)c12 |t:1| Show InChI InChI=1S/C19H16F2N6O/c20-13-3-4-14(21)16-15(13)17(23)26-19(25-16)5-7-27(8-6-19)18(28)11-1-2-12(9-22)24-10-11/h1-4,10,25H,5-8H2,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 37.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418856
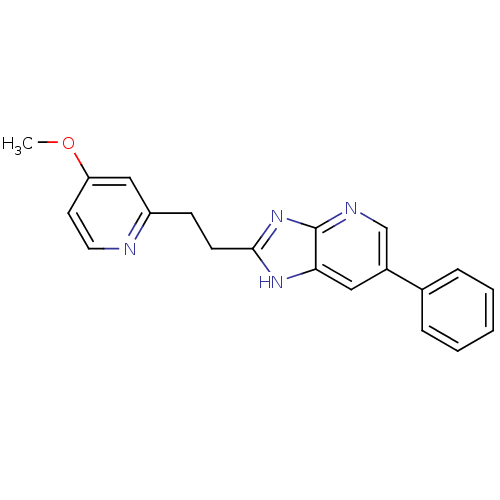
(CHEMBL1800534)Show InChI InChI=1S/C20H18N4O/c1-25-17-9-10-21-16(12-17)7-8-19-23-18-11-15(13-22-20(18)24-19)14-5-3-2-4-6-14/h2-6,9-13H,7-8H2,1H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50091805

(2-amino-4,6-dimethylpyridine | 4,6-Dimethyl-pyridi...)Show InChI InChI=1S/C7H10N2/c1-5-3-6(2)9-7(8)4-5/h3-4H,1-2H3,(H2,8,9) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418855

(CHEMBL1800533)Show InChI InChI=1S/C18H22N4O/c1-3-4-5-13-10-16-18(20-12-13)22-17(21-16)7-6-14-11-15(23-2)8-9-19-14/h8-12H,3-7H2,1-2H3,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55.0 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418844

(CHEMBL1800347)Show InChI InChI=1S/C18H19N5O2/c1-25-16-4-7-20-17(10-16)22-14-5-8-23(9-6-14)18(24)13-2-3-15(11-19)21-12-13/h2-4,7,10,12,14H,5-6,8-9H2,1H3,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418849

(CHEMBL1738840)Show InChI InChI=1S/C14H14N4O/c1-19-11-6-8-15-10(9-11)4-5-13-17-12-3-2-7-16-14(12)18-13/h2-3,6-9H,4-5H2,1H3,(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 81.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50091805

(2-amino-4,6-dimethylpyridine | 4,6-Dimethyl-pyridi...)Show InChI InChI=1S/C7H10N2/c1-5-3-6(2)9-7(8)4-5/h3-4H,1-2H3,(H2,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418845
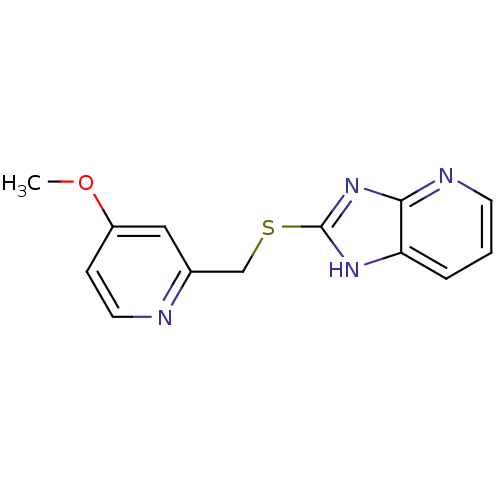
(CHEMBL1800348)Show InChI InChI=1S/C13H12N4OS/c1-18-10-4-6-14-9(7-10)8-19-13-16-11-3-2-5-15-12(11)17-13/h2-7H,8H2,1H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418851
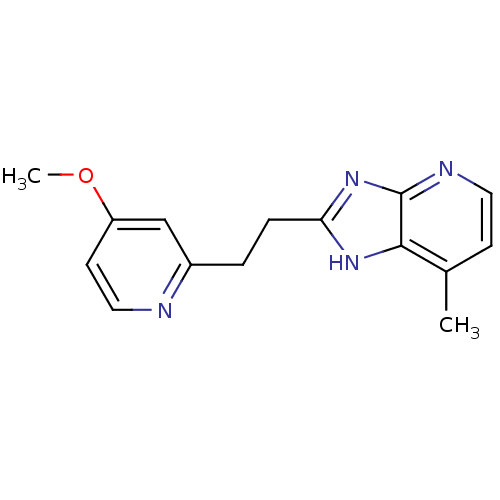
(CHEMBL1800528)Show InChI InChI=1S/C15H16N4O/c1-10-5-7-17-15-14(10)18-13(19-15)4-3-11-9-12(20-2)6-8-16-11/h5-9H,3-4H2,1-2H3,(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418847
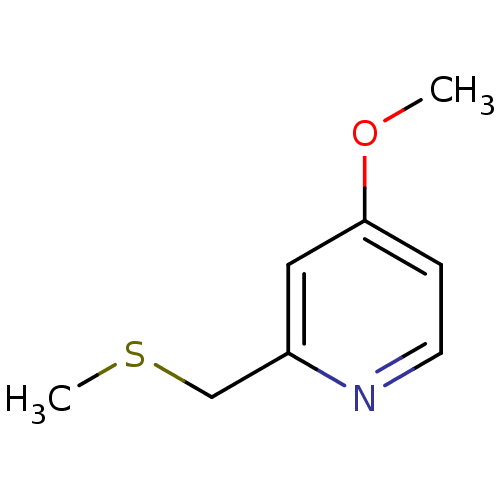
(CHEMBL1800523)Show InChI InChI=1S/C8H11NOS/c1-10-8-3-4-9-7(5-8)6-11-2/h3-5H,6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418860

(CHEMBL1800526)Show InChI InChI=1S/C14H13BrN4O/c1-20-11-4-5-16-10(7-11)2-3-13-18-12-6-9(15)8-17-14(12)19-13/h4-8H,2-3H2,1H3,(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418850
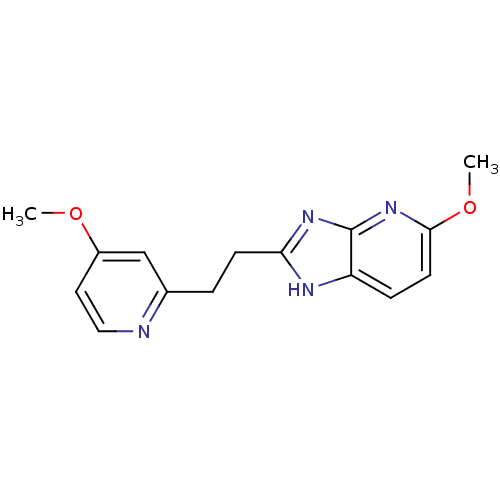
(CHEMBL1800527)Show InChI InChI=1S/C15H16N4O2/c1-20-11-7-8-16-10(9-11)3-5-13-17-12-4-6-14(21-2)19-15(12)18-13/h4,6-9H,3,5H2,1-2H3,(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50418847

(CHEMBL1800523)Show InChI InChI=1S/C8H11NOS/c1-10-8-3-4-9-7(5-8)6-11-2/h3-5H,6H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 447 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418859
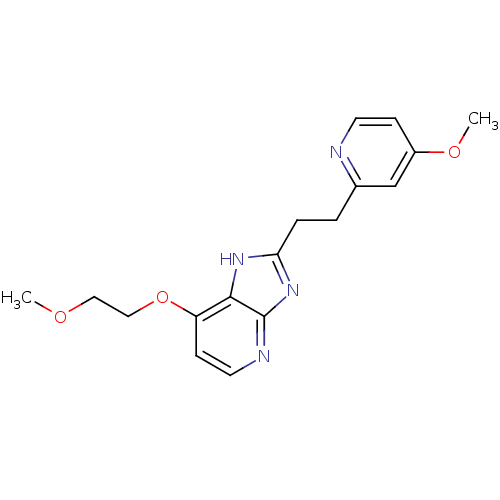
(CHEMBL1800531)Show InChI InChI=1S/C17H20N4O3/c1-22-9-10-24-14-6-8-19-17-16(14)20-15(21-17)4-3-12-11-13(23-2)5-7-18-12/h5-8,11H,3-4,9-10H2,1-2H3,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 513 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418854
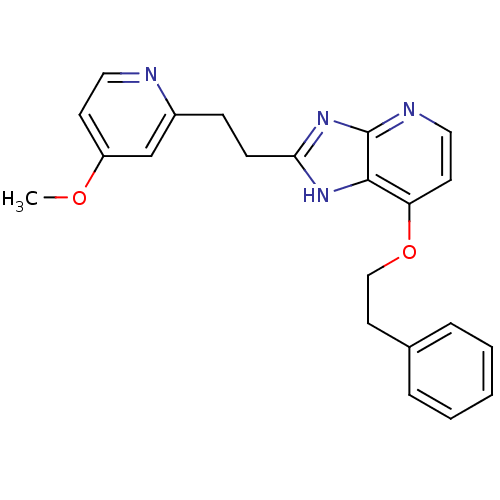
(CHEMBL1800532)Show SMILES COc1ccnc(CCc2nc3nccc(OCCc4ccccc4)c3[nH]2)c1 Show InChI InChI=1S/C22H22N4O2/c1-27-18-9-12-23-17(15-18)7-8-20-25-21-19(10-13-24-22(21)26-20)28-14-11-16-5-3-2-4-6-16/h2-6,9-10,12-13,15H,7-8,11,14H2,1H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 724 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50124535

(1-(6-CYANO-3-PYRIDYLCARBONYL)-5',8'-DIFLUOROSPIRO[...)Show SMILES NC1=NC2(CCN(CC2)C(=O)c2ccc(nc2)C#N)Nc2c(F)ccc(F)c12 |t:1| Show InChI InChI=1S/C19H16F2N6O/c20-13-3-4-14(21)16-15(13)17(23)26-19(25-16)5-7-27(8-6-19)18(28)11-1-2-12(9-22)24-10-11/h1-4,10,25H,5-8H2,(H2,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 741 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418853

(CHEMBL1800530)Show InChI InChI=1S/C15H16N4O2/c1-20-11-5-7-16-10(9-11)3-4-13-18-14-12(21-2)6-8-17-15(14)19-13/h5-9H,3-4H2,1-2H3,(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 776 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50418847

(CHEMBL1800523)Show InChI InChI=1S/C8H11NOS/c1-10-8-3-4-9-7(5-8)6-11-2/h3-5H,6H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50418856

(CHEMBL1800534)Show InChI InChI=1S/C20H18N4O/c1-25-17-9-10-21-16(12-17)7-8-19-23-18-11-15(13-22-20(18)24-19)14-5-3-2-4-6-14/h2-6,9-13H,7-8H2,1H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50086467
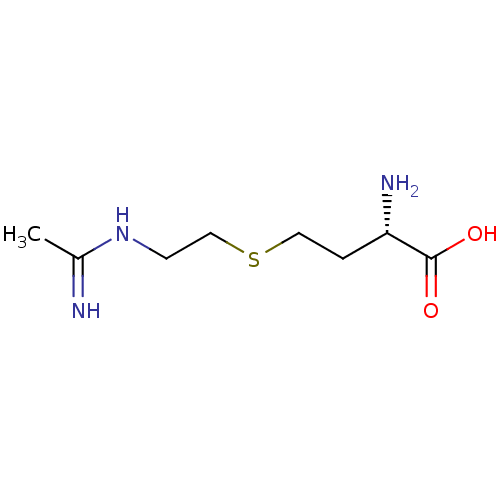
((S)-4-(2-Acetimidoylamino-ethylsulfanyl)-2-amino-b...)Show InChI InChI=1S/C8H17N3O2S/c1-6(9)11-3-5-14-4-2-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50418855

(CHEMBL1800533)Show InChI InChI=1S/C18H22N4O/c1-3-4-5-13-10-16-18(20-12-13)22-17(21-16)7-6-14-11-15(23-2)8-9-19-14/h8-12H,3-7H2,1-2H3,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418852

(CHEMBL1800529)Show InChI InChI=1S/C16H18N4O/c1-10-8-11(2)18-16-15(10)19-14(20-16)5-4-12-9-13(21-3)6-7-17-12/h6-9H,4-5H2,1-3H3,(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50418849

(CHEMBL1738840)Show InChI InChI=1S/C14H14N4O/c1-19-11-6-8-15-10(9-11)4-5-13-17-12-3-2-7-16-14(12)18-13/h2-3,6-9H,4-5H2,1H3,(H,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50418845

(CHEMBL1800348)Show InChI InChI=1S/C13H12N4OS/c1-18-10-4-6-14-9(7-10)8-19-13-16-11-3-2-5-15-12(11)17-13/h2-7H,8H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50063300
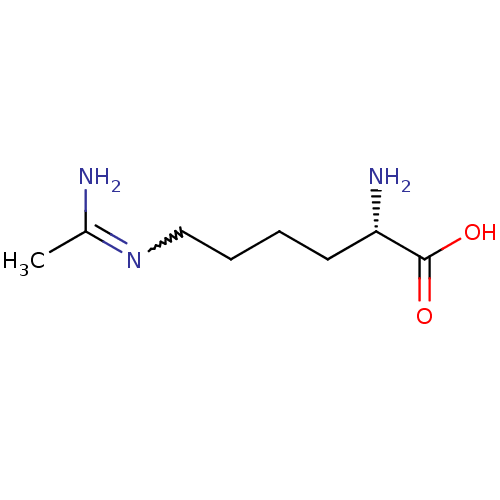
((L-N6-1-iminoethyl)lysine | (S)-6-Acetimidoylamino...)Show InChI InChI=1S/C8H17N3O2/c1-6(9)11-5-3-2-4-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50418844

(CHEMBL1800347)Show InChI InChI=1S/C18H19N5O2/c1-25-16-4-7-20-17(10-16)22-14-5-8-23(9-6-14)18(24)13-2-3-15(11-19)21-12-13/h2-4,7,10,12,14H,5-6,8-9H2,1H3,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50418860

(CHEMBL1800526)Show InChI InChI=1S/C14H13BrN4O/c1-20-11-4-5-16-10(7-11)2-3-13-18-12-6-9(15)8-17-14(12)19-13/h4-8H,2-3H2,1H3,(H,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418846

(CHEMBL51478)Show InChI InChI=1S/C14H13N3OS/c1-18-11-6-7-15-10(8-11)9-19-14-16-12-4-2-3-5-13(12)17-14/h2-8H,9H2,1H3,(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50418852

(CHEMBL1800529)Show InChI InChI=1S/C16H18N4O/c1-10-8-11(2)18-16-15(10)19-14(20-16)5-4-12-9-13(21-3)6-7-17-12/h6-9H,4-5H2,1-3H3,(H,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50418854

(CHEMBL1800532)Show SMILES COc1ccnc(CCc2nc3nccc(OCCc4ccccc4)c3[nH]2)c1 Show InChI InChI=1S/C22H22N4O2/c1-27-18-9-12-23-17(15-18)7-8-20-25-21-19(10-13-24-22(21)26-20)28-14-11-16-5-3-2-4-6-16/h2-6,9-10,12-13,15H,7-8,11,14H2,1H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50418849

(CHEMBL1738840)Show InChI InChI=1S/C14H14N4O/c1-19-11-6-8-15-10(9-11)4-5-13-17-12-3-2-7-16-14(12)18-13/h2-3,6-9H,4-5H2,1H3,(H,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50418856

(CHEMBL1800534)Show InChI InChI=1S/C20H18N4O/c1-25-17-9-10-21-16(12-17)7-8-19-23-18-11-15(13-22-20(18)24-19)14-5-3-2-4-6-14/h2-6,9-13H,7-8H2,1H3,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50418851

(CHEMBL1800528)Show InChI InChI=1S/C15H16N4O/c1-10-5-7-17-15-14(10)18-13(19-15)4-3-11-9-12(20-2)6-8-16-11/h5-9H,3-4H2,1-2H3,(H,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50418850

(CHEMBL1800527)Show InChI InChI=1S/C15H16N4O2/c1-20-11-7-8-16-10(9-11)3-5-13-17-12-4-6-14(21-2)19-15(12)18-13/h4,6-9H,3,5H2,1-2H3,(H,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50418846

(CHEMBL51478)Show InChI InChI=1S/C14H13N3OS/c1-18-11-6-7-15-10(8-11)9-19-14-16-12-4-2-3-5-13(12)17-14/h2-8H,9H2,1H3,(H,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50418846

(CHEMBL51478)Show InChI InChI=1S/C14H13N3OS/c1-18-11-6-7-15-10(8-11)9-19-14-16-12-4-2-3-5-13(12)17-14/h2-8H,9H2,1H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50418851

(CHEMBL1800528)Show InChI InChI=1S/C15H16N4O/c1-10-5-7-17-15-14(10)18-13(19-15)4-3-11-9-12(20-2)6-8-16-11/h5-9H,3-4H2,1-2H3,(H,17,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418857
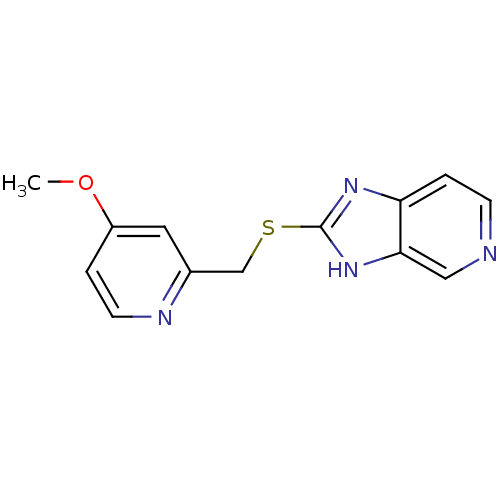
(CHEMBL1800349)Show InChI InChI=1S/C13H12N4OS/c1-18-10-2-5-15-9(6-10)8-19-13-16-11-3-4-14-7-12(11)17-13/h2-7H,8H2,1H3,(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50418845

(CHEMBL1800348)Show InChI InChI=1S/C13H12N4OS/c1-18-10-4-6-14-9(7-10)8-19-13-16-11-3-2-5-15-12(11)17-13/h2-7H,8H2,1H3,(H,15,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50418853

(CHEMBL1800530)Show InChI InChI=1S/C15H16N4O2/c1-20-11-5-7-16-10(9-11)3-4-13-18-14-12(21-2)6-8-17-15(14)19-13/h5-9H,3-4H2,1-2H3,(H,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50063300

((L-N6-1-iminoethyl)lysine | (S)-6-Acetimidoylamino...)Show InChI InChI=1S/C8H17N3O2/c1-6(9)11-5-3-2-4-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50418854

(CHEMBL1800532)Show SMILES COc1ccnc(CCc2nc3nccc(OCCc4ccccc4)c3[nH]2)c1 Show InChI InChI=1S/C22H22N4O2/c1-27-18-9-12-23-17(15-18)7-8-20-25-21-19(10-13-24-22(21)26-20)28-14-11-16-5-3-2-4-6-16/h2-6,9-10,12-13,15H,7-8,11,14H2,1H3,(H,24,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50418855

(CHEMBL1800533)Show InChI InChI=1S/C18H22N4O/c1-3-4-5-13-10-16-18(20-12-13)22-17(21-16)7-6-14-11-15(23-2)8-9-19-14/h8-12H,3-7H2,1-2H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50418860

(CHEMBL1800526)Show InChI InChI=1S/C14H13BrN4O/c1-20-11-4-5-16-10(7-11)2-3-13-18-12-6-9(15)8-17-14(12)19-13/h4-8H,2-3H2,1H3,(H,17,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50418858

(CHEMBL1800524)Show InChI InChI=1S/C9H13NO2S/c1-11-8-4-7(6-13-3)10-5-9(8)12-2/h4-5H,6H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50418848

(CHEMBL1800525)Show InChI InChI=1S/C9H13NO2S/c1-11-8-4-5-10-7(6-13-3)9(8)12-2/h4-5H,6H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50418844

(CHEMBL1800347)Show InChI InChI=1S/C18H19N5O2/c1-25-16-4-7-20-17(10-16)22-14-5-8-23(9-6-14)18(24)13-2-3-15(11-19)21-12-13/h2-4,7,10,12,14H,5-6,8-9H2,1H3,(H,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418843

(CHEMBL1185460)Show SMILES CC(N)=NCCCC[C@H](N)C(=O)Nc1nnn[nH]1 |r,w:3.3| Show InChI InChI=1S/C9H18N8O/c1-6(10)12-5-3-2-4-7(11)8(18)13-9-14-16-17-15-9/h7H,2-5,11H2,1H3,(H2,10,12)(H2,13,14,15,16,17,18)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50124535

(1-(6-CYANO-3-PYRIDYLCARBONYL)-5',8'-DIFLUOROSPIRO[...)Show SMILES NC1=NC2(CCN(CC2)C(=O)c2ccc(nc2)C#N)Nc2c(F)ccc(F)c12 |t:1| Show InChI InChI=1S/C19H16F2N6O/c20-13-3-4-14(21)16-15(13)17(23)26-19(25-16)5-7-27(8-6-19)18(28)11-1-2-12(9-22)24-10-11/h1-4,10,25H,5-8H2,(H2,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50418857

(CHEMBL1800349)Show InChI InChI=1S/C13H12N4OS/c1-18-10-2-5-15-9(6-10)8-19-13-16-11-3-4-14-7-12(11)17-13/h2-7H,8H2,1H3,(H,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50418850

(CHEMBL1800527)Show InChI InChI=1S/C15H16N4O2/c1-20-11-7-8-16-10(9-11)3-5-13-17-12-4-6-14(21-2)19-15(12)18-13/h4,6-9H,3,5H2,1-2H3,(H,17,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50418848

(CHEMBL1800525)Show InChI InChI=1S/C9H13NO2S/c1-11-8-4-5-10-7(6-13-3)9(8)12-2/h4-5H,6H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50418843

(CHEMBL1185460)Show SMILES CC(N)=NCCCC[C@H](N)C(=O)Nc1nnn[nH]1 |r,w:3.3| Show InChI InChI=1S/C9H18N8O/c1-6(10)12-5-3-2-4-7(11)8(18)13-9-14-16-17-15-9/h7H,2-5,11H2,1H3,(H2,10,12)(H2,13,14,15,16,17,18)/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50418843

(CHEMBL1185460)Show SMILES CC(N)=NCCCC[C@H](N)C(=O)Nc1nnn[nH]1 |r,w:3.3| Show InChI InChI=1S/C9H18N8O/c1-6(10)12-5-3-2-4-7(11)8(18)13-9-14-16-17-15-9/h7H,2-5,11H2,1H3,(H2,10,12)(H2,13,14,15,16,17,18)/t7-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50418852

(CHEMBL1800529)Show InChI InChI=1S/C16H18N4O/c1-10-8-11(2)18-16-15(10)19-14(20-16)5-4-12-9-13(21-3)6-7-17-12/h6-9H,4-5H2,1-3H3,(H,18,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418858

(CHEMBL1800524)Show InChI InChI=1S/C9H13NO2S/c1-11-8-4-7(6-13-3)10-5-9(8)12-2/h4-5H,6H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50418858

(CHEMBL1800524)Show InChI InChI=1S/C9H13NO2S/c1-11-8-4-7(6-13-3)10-5-9(8)12-2/h4-5H,6H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418848

(CHEMBL1800525)Show InChI InChI=1S/C9H13NO2S/c1-11-8-4-5-10-7(6-13-3)9(8)12-2/h4-5H,6H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50063300

((L-N6-1-iminoethyl)lysine | (S)-6-Acetimidoylamino...)Show InChI InChI=1S/C8H17N3O2/c1-6(9)11-5-3-2-4-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50418857

(CHEMBL1800349)Show InChI InChI=1S/C13H12N4OS/c1-18-10-2-5-15-9(6-10)8-19-13-16-11-3-4-14-7-12(11)17-13/h2-7H,8H2,1H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50418849

(CHEMBL1738840)Show InChI InChI=1S/C14H14N4O/c1-19-11-6-8-15-10(9-11)4-5-13-17-12-3-2-7-16-14(12)18-13/h2-3,6-9H,4-5H2,1H3,(H,16,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50418859

(CHEMBL1800531)Show InChI InChI=1S/C17H20N4O3/c1-22-9-10-24-14-6-8-19-17-16(14)20-15(21-17)4-3-12-11-13(23-2)5-7-18-12/h5-8,11H,3-4,9-10H2,1-2H3,(H,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.23E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50086467

((S)-4-(2-Acetimidoylamino-ethylsulfanyl)-2-amino-b...)Show InChI InChI=1S/C8H17N3O2S/c1-6(9)11-3-5-14-4-2-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50418859

(CHEMBL1800531)Show InChI InChI=1S/C17H20N4O3/c1-22-9-10-24-14-6-8-19-17-16(14)20-15(21-17)4-3-12-11-13(23-2)5-7-18-12/h5-8,11H,3-4,9-10H2,1-2H3,(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50418853

(CHEMBL1800530)Show InChI InChI=1S/C15H16N4O2/c1-20-11-5-7-16-10(9-11)3-4-13-18-14-12(21-2)6-8-17-15(14)19-13/h5-9H,3-4H2,1-2H3,(H,17,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50086467

((S)-4-(2-Acetimidoylamino-ethylsulfanyl)-2-amino-b...)Show InChI InChI=1S/C8H17N3O2S/c1-6(9)11-3-5-14-4-2-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.68E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data