 Found 22 hits of Enzyme Inhibition Constant Data
Found 22 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50392045
(CHEMBL2152561)Show InChI InChI=1S/C16H14O4S/c17-15(18)12-5-1-4-11(9-12)13-6-2-3-10(7-8-21)14(13)16(19)20/h1-6,9,21H,7-8H2,(H,17,18)(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
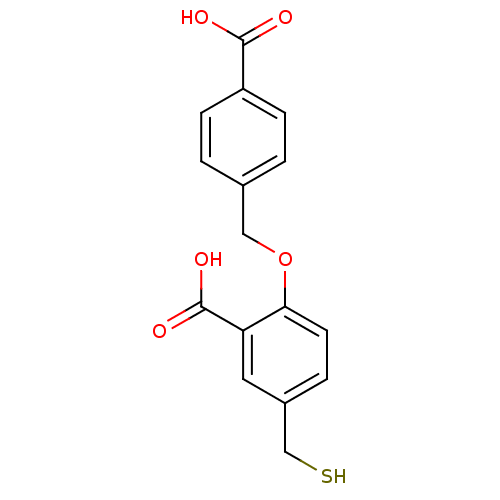
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50392040
(CHEMBL2152556)Show InChI InChI=1S/C16H14O5S/c17-15(18)12-3-1-2-10(6-12)8-21-14-5-4-11(9-22)7-13(14)16(19)20/h1-7,22H,8-9H2,(H,17,18)(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50392046
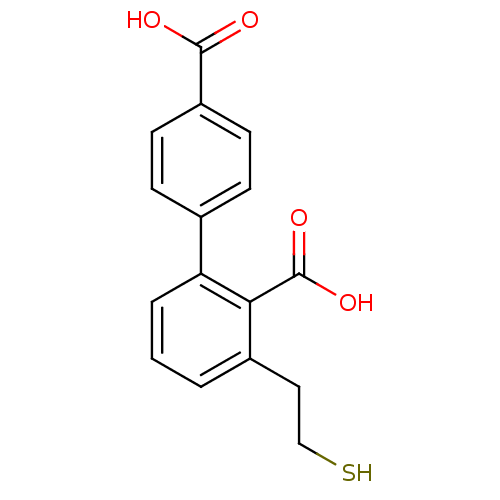
(CHEMBL2152562)Show InChI InChI=1S/C16H14O4S/c17-15(18)12-6-4-10(5-7-12)13-3-1-2-11(8-9-21)14(13)16(19)20/h1-7,21H,8-9H2,(H,17,18)(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM17762

(3-[2-carboxy-2-(3-sulfanylpropyl)ethyl]benzoic aci...)Show InChI InChI=1S/C13H16O4S/c14-12(15)10-4-1-3-9(7-10)8-11(13(16)17)5-2-6-18/h1,3-4,7,11,18H,2,5-6,8H2,(H,14,15)(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
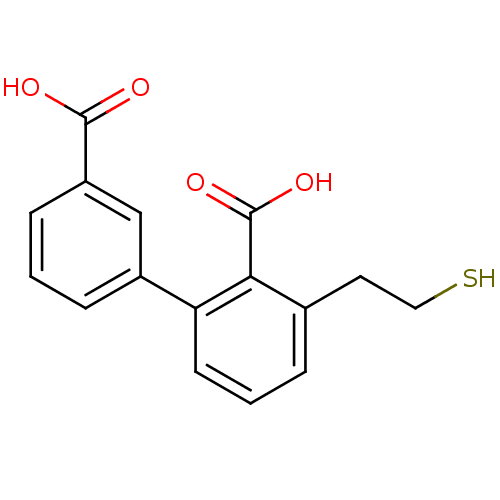
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50392041
(CHEMBL2152557)Show InChI InChI=1S/C16H14O5S/c17-15(18)12-4-1-10(2-5-12)8-21-14-6-3-11(9-22)7-13(14)16(19)20/h1-7,22H,8-9H2,(H,17,18)(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50332228
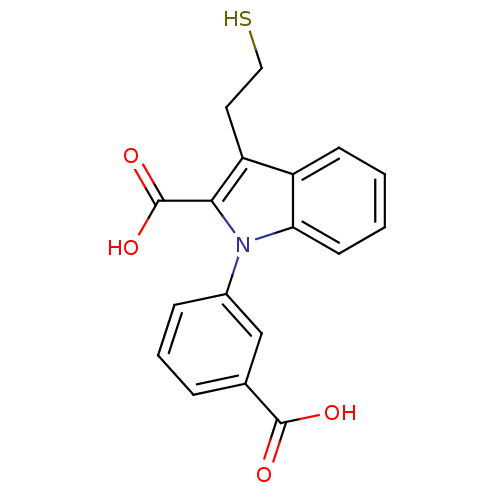
(1-(3-Carboxyphenyl)-3-(2-mercapto-ethyl)-1H-indole...)Show InChI InChI=1S/C18H15NO4S/c20-17(21)11-4-3-5-12(10-11)19-15-7-2-1-6-13(15)14(8-9-24)16(19)18(22)23/h1-7,10,24H,8-9H2,(H,20,21)(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50392036
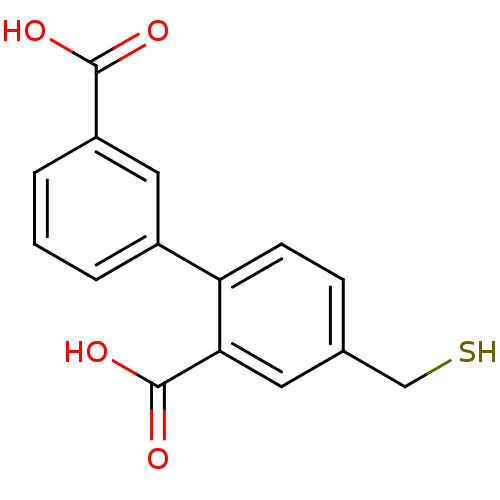
(CHEMBL2152437)Show InChI InChI=1S/C15H12O4S/c16-14(17)11-3-1-2-10(7-11)12-5-4-9(8-20)6-13(12)15(18)19/h1-7,20H,8H2,(H,16,17)(H,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
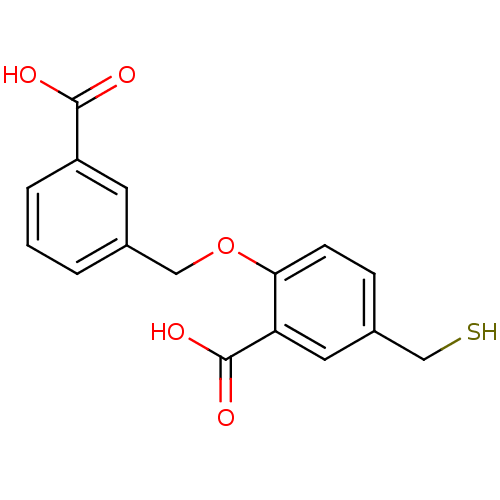
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50392039
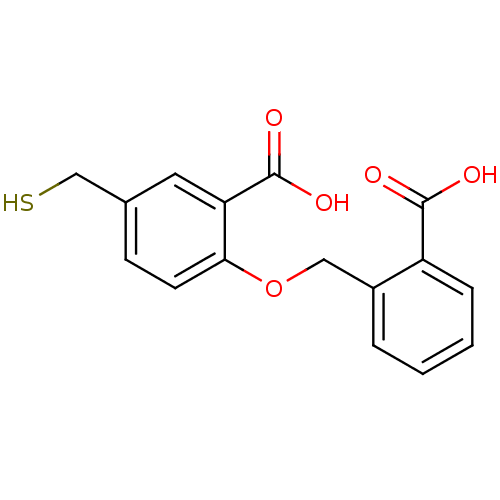
(CHEMBL2152555)Show InChI InChI=1S/C16H14O5S/c17-15(18)12-4-2-1-3-11(12)8-21-14-6-5-10(9-22)7-13(14)16(19)20/h1-7,22H,8-9H2,(H,17,18)(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50392037
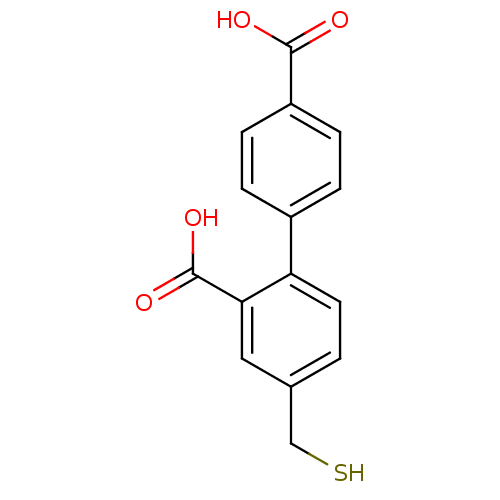
(CHEMBL2152438)Show InChI InChI=1S/C15H12O4S/c16-14(17)11-4-2-10(3-5-11)12-6-1-9(8-20)7-13(12)15(18)19/h1-7,20H,8H2,(H,16,17)(H,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50392049
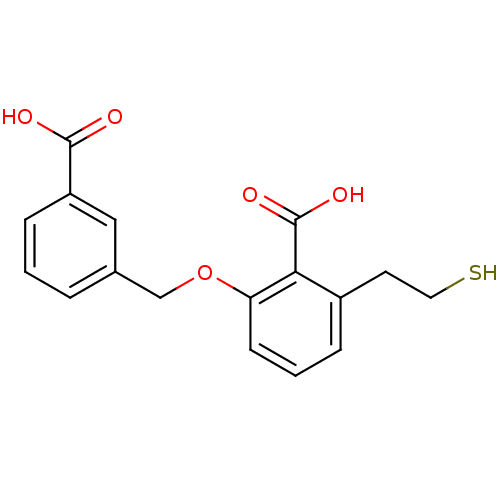
(CHEMBL2152565)Show InChI InChI=1S/C17H16O5S/c18-16(19)13-5-1-3-11(9-13)10-22-14-6-2-4-12(7-8-23)15(14)17(20)21/h1-6,9,23H,7-8,10H2,(H,18,19)(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM17755
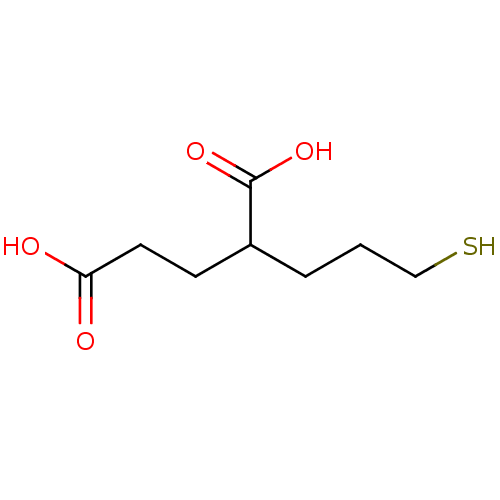
(2-(3-sulfanylpropyl)pentanedioic acid | 2-MPPA | C...)Show InChI InChI=1S/C8H14O4S/c9-7(10)4-3-6(8(11)12)2-1-5-13/h6,13H,1-5H2,(H,9,10)(H,11,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50392048
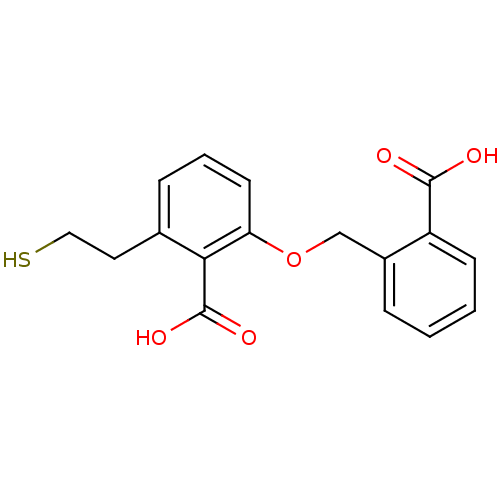
(CHEMBL2152564)Show InChI InChI=1S/C17H16O5S/c18-16(19)13-6-2-1-4-12(13)10-22-14-7-3-5-11(8-9-23)15(14)17(20)21/h1-7,23H,8-10H2,(H,18,19)(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50392050
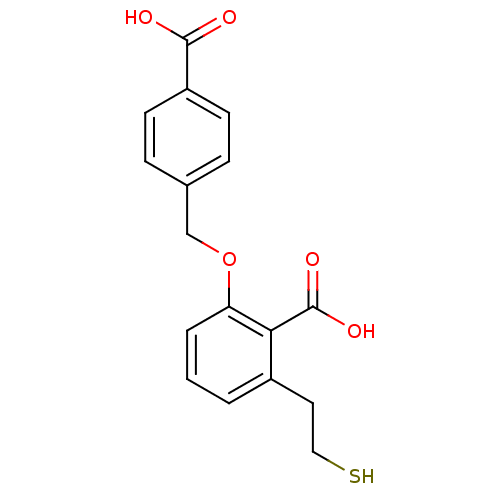
(CHEMBL2152566)Show InChI InChI=1S/C17H16O5S/c18-16(19)13-6-4-11(5-7-13)10-22-14-3-1-2-12(8-9-23)15(14)17(20)21/h1-7,23H,8-10H2,(H,18,19)(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50392047
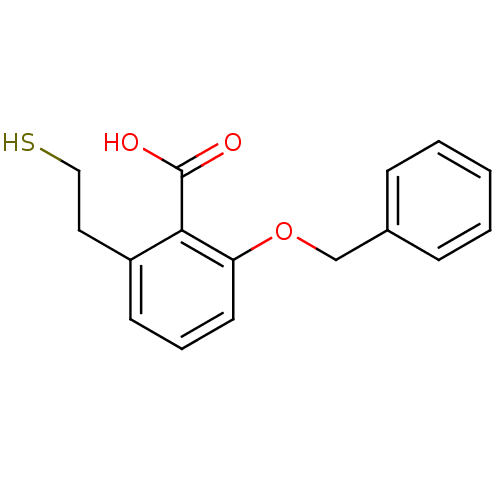
(CHEMBL2152563)Show InChI InChI=1S/C16H16O3S/c17-16(18)15-13(9-10-20)7-4-8-14(15)19-11-12-5-2-1-3-6-12/h1-8,20H,9-11H2,(H,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50392044
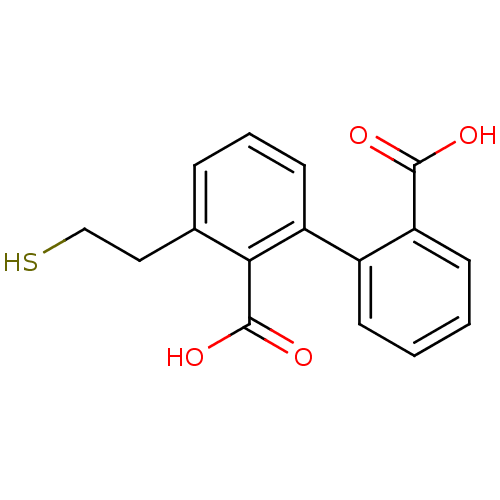
(CHEMBL2152560)Show InChI InChI=1S/C16H14O4S/c17-15(18)13-6-2-1-5-11(13)12-7-3-4-10(8-9-21)14(12)16(19)20/h1-7,21H,8-9H2,(H,17,18)(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50392035
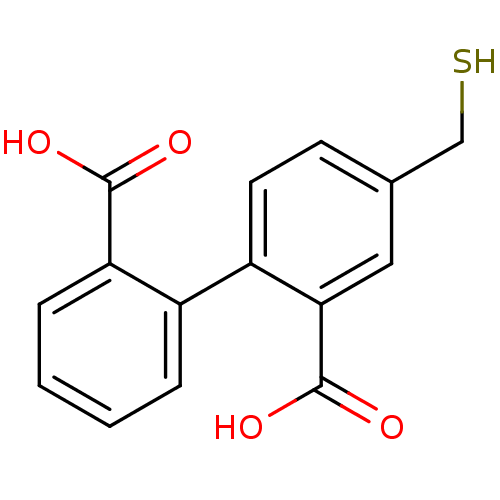
(CHEMBL2152436)Show InChI InChI=1S/C15H12O4S/c16-14(17)12-4-2-1-3-10(12)11-6-5-9(8-20)7-13(11)15(18)19/h1-7,20H,8H2,(H,16,17)(H,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50392038
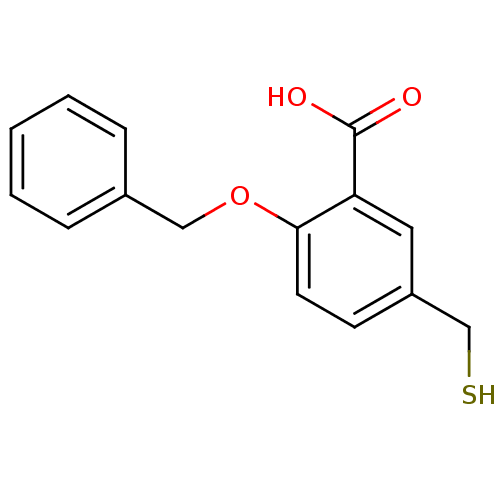
(CHEMBL2152439)Show InChI InChI=1S/C15H14O3S/c16-15(17)13-8-12(10-19)6-7-14(13)18-9-11-4-2-1-3-5-11/h1-8,19H,9-10H2,(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50392042
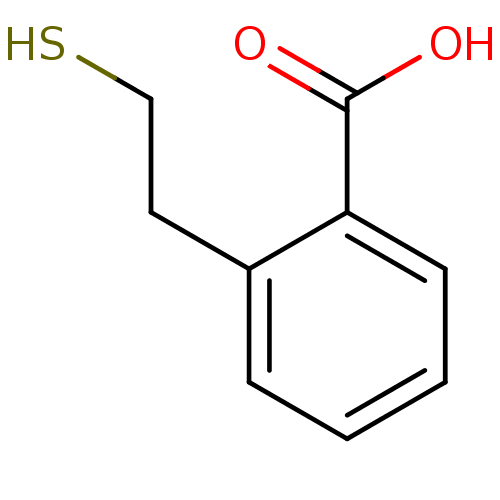
(CHEMBL2152558)Show InChI InChI=1S/C9H10O2S/c10-9(11)8-4-2-1-3-7(8)5-6-12/h1-4,12H,5-6H2,(H,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50392051
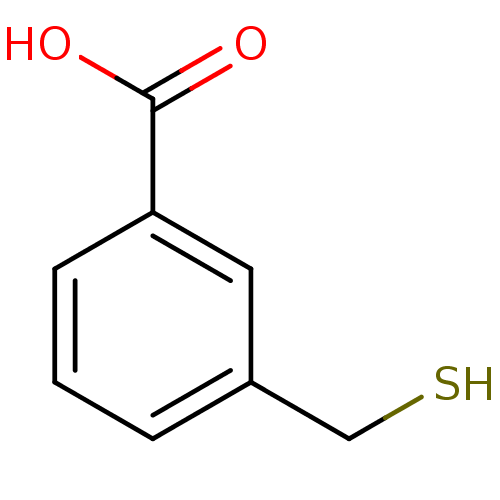
(CHEMBL2152434)Show InChI InChI=1S/C8H8O2S/c9-8(10)7-3-1-2-6(4-7)5-11/h1-4,11H,5H2,(H,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50392043
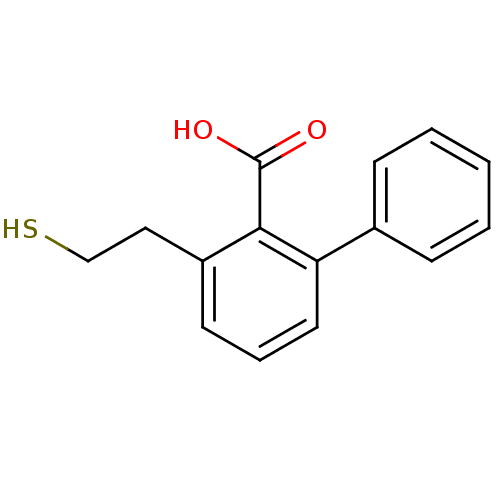
(CHEMBL2152559)Show InChI InChI=1S/C15H14O2S/c16-15(17)14-12(9-10-18)7-4-8-13(14)11-5-2-1-3-6-11/h1-8,18H,9-10H2,(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50127619
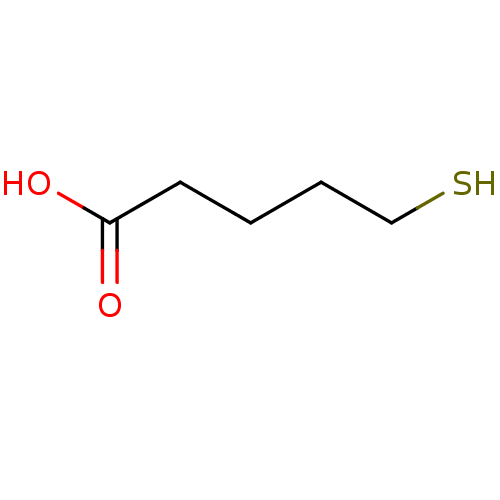
(5-Mercapto-pentanoic acid | CHEMBL294565)Show InChI InChI=1S/C5H10O2S/c6-5(7)3-1-2-4-8/h8H,1-4H2,(H,6,7) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50392034
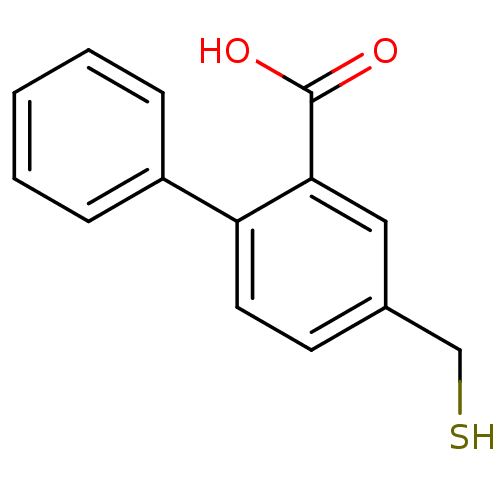
(CHEMBL2152435)Show InChI InChI=1S/C14H12O2S/c15-14(16)13-8-10(9-17)6-7-12(13)11-4-2-1-3-5-11/h1-8,17H,9H2,(H,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay |
J Med Chem 55: 5922-32 (2012)
Article DOI: 10.1021/jm300488m
BindingDB Entry DOI: 10.7270/Q21J9BWN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data