 Found 12 hits of Enzyme Inhibition Constant Data
Found 12 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholine-binding protein
(Lymnaea stagnalis) | BDBM50382466
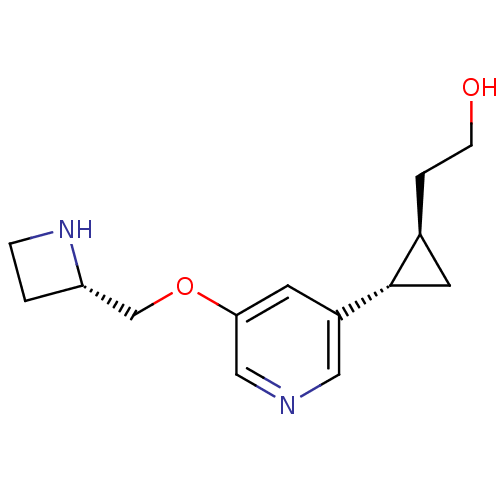
(CHEMBL2024087)Show SMILES OCC[C@H]1C[C@@H]1c1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C14H20N2O2/c17-4-2-10-6-14(10)11-5-13(8-15-7-11)18-9-12-1-3-16-12/h5,7-8,10,12,14,16-17H,1-4,6,9H2/t10-,12-,14-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 12.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from Lymnaea stagnalis AChBP |
J Med Chem 55: 8028-37 (2012)
Article DOI: 10.1021/jm3008739
BindingDB Entry DOI: 10.7270/Q2NC62BW |
More data for this
Ligand-Target Pair | |
Acetylcholine-binding protein
(Lymnaea stagnalis) | BDBM50382467
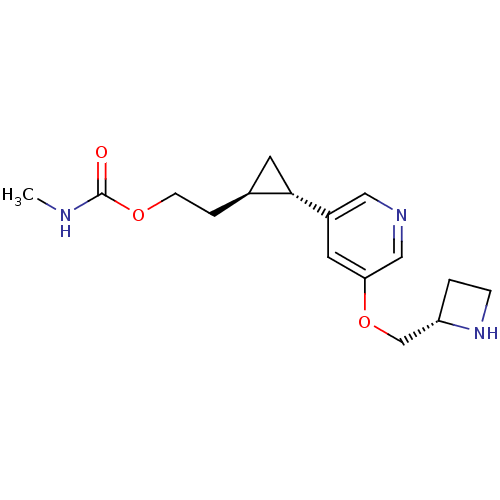
(CHEMBL2024089)Show SMILES CNC(=O)OCC[C@H]1C[C@@H]1c1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C16H23N3O3/c1-17-16(20)21-5-3-11-7-15(11)12-6-14(9-18-8-12)22-10-13-2-4-19-13/h6,8-9,11,13,15,19H,2-5,7,10H2,1H3,(H,17,20)/t11-,13-,15-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from Lymnaea stagnalis AChBP |
J Med Chem 55: 8028-37 (2012)
Article DOI: 10.1021/jm3008739
BindingDB Entry DOI: 10.7270/Q2NC62BW |
More data for this
Ligand-Target Pair | |
Acetylcholine-binding protein
(Lymnaea stagnalis) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 496 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from Lymnaea stagnalis AChBP |
J Med Chem 55: 8028-37 (2012)
Article DOI: 10.1021/jm3008739
BindingDB Entry DOI: 10.7270/Q2NC62BW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Soluble acetylcholine receptor
(Aplysia Californica) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 598 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from Aplysia californica AChBP |
J Med Chem 55: 8028-37 (2012)
Article DOI: 10.1021/jm3008739
BindingDB Entry DOI: 10.7270/Q2NC62BW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Soluble acetylcholine receptor
(Aplysia Californica) | BDBM50382466

(CHEMBL2024087)Show SMILES OCC[C@H]1C[C@@H]1c1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C14H20N2O2/c17-4-2-10-6-14(10)11-5-13(8-15-7-11)18-9-12-1-3-16-12/h5,7-8,10,12,14,16-17H,1-4,6,9H2/t10-,12-,14-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 979 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from Aplysia californica AChBP |
J Med Chem 55: 8028-37 (2012)
Article DOI: 10.1021/jm3008739
BindingDB Entry DOI: 10.7270/Q2NC62BW |
More data for this
Ligand-Target Pair | |
Soluble acetylcholine receptor
(Aplysia Californica) | BDBM50382467

(CHEMBL2024089)Show SMILES CNC(=O)OCC[C@H]1C[C@@H]1c1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C16H23N3O3/c1-17-16(20)21-5-3-11-7-15(11)12-6-14(9-18-8-12)22-10-13-2-4-19-13/h6,8-9,11,13,15,19H,2-5,7,10H2,1H3,(H,17,20)/t11-,13-,15-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from Aplysia californica AChBP |
J Med Chem 55: 8028-37 (2012)
Article DOI: 10.1021/jm3008739
BindingDB Entry DOI: 10.7270/Q2NC62BW |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398321
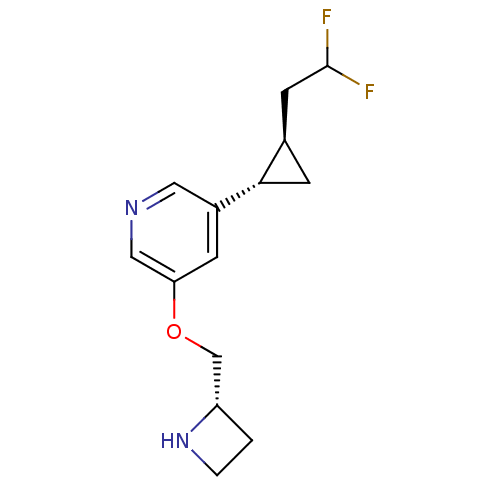
(CHEMBL2177344)Show SMILES FC(F)C[C@H]1C[C@@H]1c1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C14H18F2N2O/c15-14(16)5-9-4-13(9)10-3-12(7-17-6-10)19-8-11-1-2-18-11/h3,6-7,9,11,13-14,18H,1-2,4-5,8H2/t9-,11+,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Antagonist activity at high sensitivity and low sensitivity alpha4beta2-nAChR expressed in human SH-EP1 cells assessed as inhibition of carbamylcholi... |
J Med Chem 55: 8028-37 (2012)
Article DOI: 10.1021/jm3008739
BindingDB Entry DOI: 10.7270/Q2NC62BW |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398320
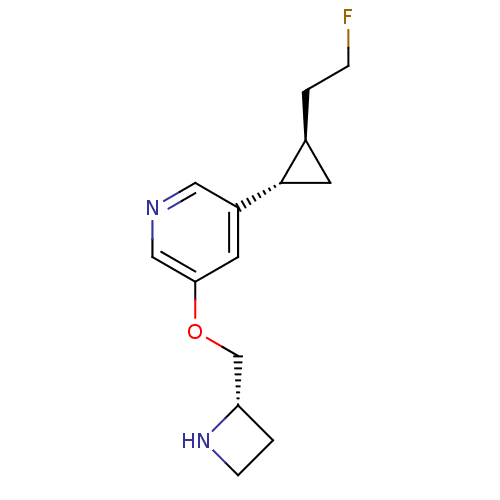
(CHEMBL2177345)Show SMILES FCC[C@H]1C[C@@H]1c1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C14H19FN2O/c15-3-1-10-6-14(10)11-5-13(8-16-7-11)18-9-12-2-4-17-12/h5,7-8,10,12,14,17H,1-4,6,9H2/t10-,12-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Antagonist activity at high sensitivity and low sensitivity alpha4beta2-nAChR expressed in human SH-EP1 cells assessed as inhibition of carbamylcholi... |
J Med Chem 55: 8028-37 (2012)
Article DOI: 10.1021/jm3008739
BindingDB Entry DOI: 10.7270/Q2NC62BW |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398319

(CHEMBL2177346)Show SMILES CC[C@H]1C[C@@H]1c1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C14H20N2O/c1-2-10-6-14(10)11-5-13(8-15-7-11)17-9-12-3-4-16-12/h5,7-8,10,12,14,16H,2-4,6,9H2,1H3/t10-,12-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22.2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Antagonist activity at high sensitivity and low sensitivity alpha4beta2-nAChR expressed in human SH-EP1 cells assessed as inhibition of carbamylcholi... |
J Med Chem 55: 8028-37 (2012)
Article DOI: 10.1021/jm3008739
BindingDB Entry DOI: 10.7270/Q2NC62BW |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398320

(CHEMBL2177345)Show SMILES FCC[C@H]1C[C@@H]1c1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C14H19FN2O/c15-3-1-10-6-14(10)11-5-13(8-16-7-11)18-9-12-2-4-17-12/h5,7-8,10,12,14,17H,1-4,6,9H2/t10-,12-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 11.3 | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Agonist activity at high sensitivity and low sensitivity alpha4beta2-nAChR expressed in human SH-EP1 cells by 86Rb+ ion flux assay |
J Med Chem 55: 8028-37 (2012)
Article DOI: 10.1021/jm3008739
BindingDB Entry DOI: 10.7270/Q2NC62BW |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398321

(CHEMBL2177344)Show SMILES FC(F)C[C@H]1C[C@@H]1c1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C14H18F2N2O/c15-14(16)5-9-4-13(9)10-3-12(7-17-6-10)19-8-11-1-2-18-11/h3,6-7,9,11,13-14,18H,1-2,4-5,8H2/t9-,11+,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Agonist activity at high sensitivity and low sensitivity alpha4beta2-nAChR expressed in human SH-EP1 cells by 86Rb+ ion flux assay |
J Med Chem 55: 8028-37 (2012)
Article DOI: 10.1021/jm3008739
BindingDB Entry DOI: 10.7270/Q2NC62BW |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398319

(CHEMBL2177346)Show SMILES CC[C@H]1C[C@@H]1c1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C14H20N2O/c1-2-10-6-14(10)11-5-13(8-15-7-11)17-9-12-3-4-16-12/h5,7-8,10,12,14,16H,2-4,6,9H2,1H3/t10-,12-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 19.1 | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Agonist activity at high sensitivity and low sensitivity alpha4beta2-nAChR expressed in human SH-EP1 cells by 86Rb+ ion flux assay |
J Med Chem 55: 8028-37 (2012)
Article DOI: 10.1021/jm3008739
BindingDB Entry DOI: 10.7270/Q2NC62BW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data