

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Mus musculus) | BDBM50008294 (2-(4-(((2-amino-4-oxo-1,4-dihydroquinazolin-6-yl)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The compound was not tested for inhibition of L1210 Thymidylate synthase due to insolubility | J Med Chem 33: 2045-51 (1990) BindingDB Entry DOI: 10.7270/Q26Q1XVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50011243 (2-{4-[(4-Hydroxy-quinazolin-6-ylmethyl)-prop-2-yny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The compound was not tested for inhibition of L1210 Thymidylate synthase due to insolubility | J Med Chem 33: 2045-51 (1990) BindingDB Entry DOI: 10.7270/Q26Q1XVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50015707 (2-Amino-6-({prop-2-ynyl-[4-(2,2,2-trifluoro-acetyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The compound was not tested for inhibition of L1210 Thymidylate synthase due to insolubility | J Med Chem 33: 2045-51 (1990) BindingDB Entry DOI: 10.7270/Q26Q1XVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50015708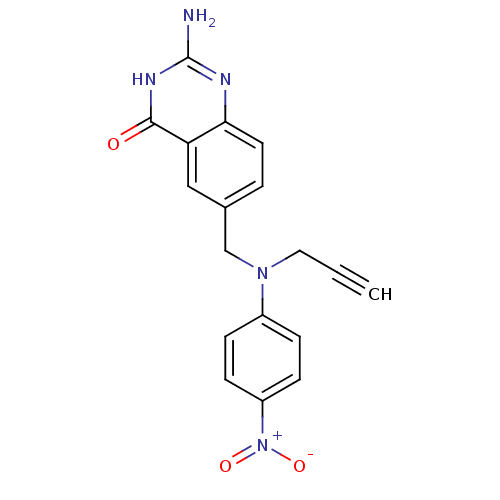 (2-Amino-6-{[(4-nitro-phenyl)-prop-2-ynyl-amino]-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The compound was not tested for inhibition of L1210 Thymidylate synthase due to insolubility | J Med Chem 33: 2045-51 (1990) BindingDB Entry DOI: 10.7270/Q26Q1XVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50015702 (4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylmethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The compound was not tested for inhibition of L1210 Thymidylate synthase due to insolubility | J Med Chem 33: 2045-51 (1990) BindingDB Entry DOI: 10.7270/Q26Q1XVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50015701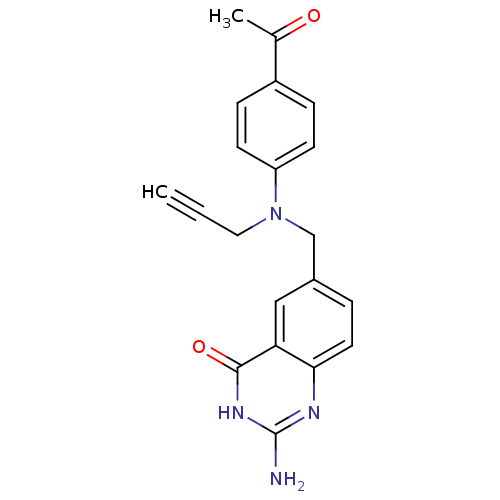 (6-{[(4-Acetyl-phenyl)-prop-2-ynyl-amino]-methyl}-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The compound was not tested for inhibition of L1210 Thymidylate synthase due to insolubility | J Med Chem 33: 2045-51 (1990) BindingDB Entry DOI: 10.7270/Q26Q1XVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50015697 (4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylmethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The compound was not tested for inhibition of L1210 Thymidylate synthase due to insolubility | J Med Chem 33: 2045-51 (1990) BindingDB Entry DOI: 10.7270/Q26Q1XVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50015703 (4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylmethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The compound was not tested for inhibition of L1210 Thymidylate synthase due to insolubility | J Med Chem 33: 2045-51 (1990) BindingDB Entry DOI: 10.7270/Q26Q1XVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50015696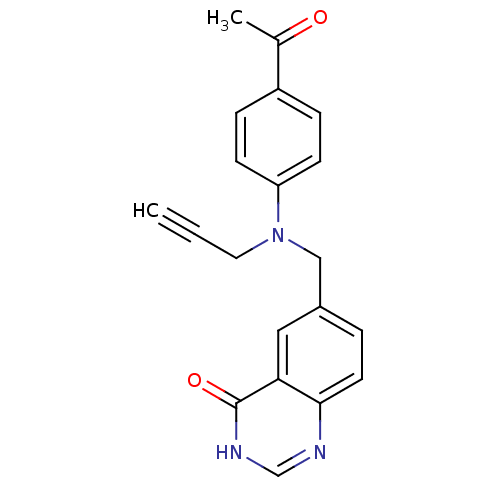 (6-{[(4-Acetyl-phenyl)-prop-2-ynyl-amino]-methyl}-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The compound was not tested for inhibition of L1210 Thymidylate synthase due to insolubility | J Med Chem 33: 2045-51 (1990) BindingDB Entry DOI: 10.7270/Q26Q1XVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50008294 (2-(4-(((2-amino-4-oxo-1,4-dihydroquinazolin-6-yl)m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Tested for 50% inhibition of WI-L2 Dihydrofolate reductase | J Med Chem 33: 2045-51 (1990) BindingDB Entry DOI: 10.7270/Q26Q1XVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50015707 (2-Amino-6-({prop-2-ynyl-[4-(2,2,2-trifluoro-acetyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Tested for 50% inhibition of WI-L2 Dihydrofolate reductase | J Med Chem 33: 2045-51 (1990) BindingDB Entry DOI: 10.7270/Q26Q1XVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50368120 (CHEMBL62672) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The compound was not tested for inhibition of L1210 Thymidylate synthase due to insolubility | J Med Chem 33: 2045-51 (1990) BindingDB Entry DOI: 10.7270/Q26Q1XVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50368121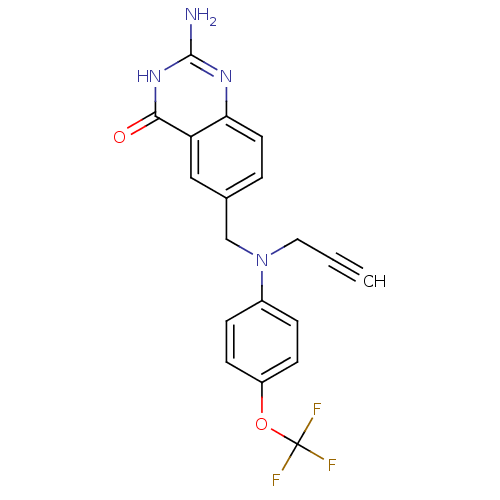 (CHEMBL293442) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The compound was not tested for inhibition of L1210 Thymidylate synthase due to insolubility | J Med Chem 33: 2045-51 (1990) BindingDB Entry DOI: 10.7270/Q26Q1XVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50368123 (CHEMBL64776) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The compound was not tested for inhibition of L1210 Thymidylate synthase due to insolubility | J Med Chem 33: 2045-51 (1990) BindingDB Entry DOI: 10.7270/Q26Q1XVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50015697 (4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylmethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Tested for 50% inhibition of WI-L2 Dihydrofolate reductase | J Med Chem 33: 2045-51 (1990) BindingDB Entry DOI: 10.7270/Q26Q1XVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50015708 (2-Amino-6-{[(4-nitro-phenyl)-prop-2-ynyl-amino]-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Tested for 50% inhibition of WI-L2 Dihydrofolate reductase | J Med Chem 33: 2045-51 (1990) BindingDB Entry DOI: 10.7270/Q26Q1XVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50015701 (6-{[(4-Acetyl-phenyl)-prop-2-ynyl-amino]-methyl}-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Tested for 50% inhibition of WI-L2 Dihydrofolate reductase | J Med Chem 33: 2045-51 (1990) BindingDB Entry DOI: 10.7270/Q26Q1XVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50015704 (6-{[Prop-2-ynyl-(3,4,5-trimethoxy-phenyl)-amino]-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 6.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Tested for 50% inhibition of WI-L2 Dihydrofolate reductase | J Med Chem 33: 2045-51 (1990) BindingDB Entry DOI: 10.7270/Q26Q1XVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50015703 (4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylmethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Tested for 50% inhibition of WI-L2 Dihydrofolate reductase | J Med Chem 33: 2045-51 (1990) BindingDB Entry DOI: 10.7270/Q26Q1XVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50015702 (4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylmethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Tested for 50% inhibition of WI-L2 Dihydrofolate reductase | J Med Chem 33: 2045-51 (1990) BindingDB Entry DOI: 10.7270/Q26Q1XVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50015704 (6-{[Prop-2-ynyl-(3,4,5-trimethoxy-phenyl)-amino]-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The compound was not tested for inhibition of L1210 Thymidylate synthase due to insolubility | J Med Chem 33: 2045-51 (1990) BindingDB Entry DOI: 10.7270/Q26Q1XVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50368122 (CHEMBL62419) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The compound was not tested for inhibition of L1210 Thymidylate synthase due to insolubility | J Med Chem 33: 2045-51 (1990) BindingDB Entry DOI: 10.7270/Q26Q1XVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50015696 (6-{[(4-Acetyl-phenyl)-prop-2-ynyl-amino]-methyl}-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Tested for 50% inhibition of WI-L2 Dihydrofolate reductase | J Med Chem 33: 2045-51 (1990) BindingDB Entry DOI: 10.7270/Q26Q1XVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||