 Found 51 hits of Enzyme Inhibition Constant Data
Found 51 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50383684
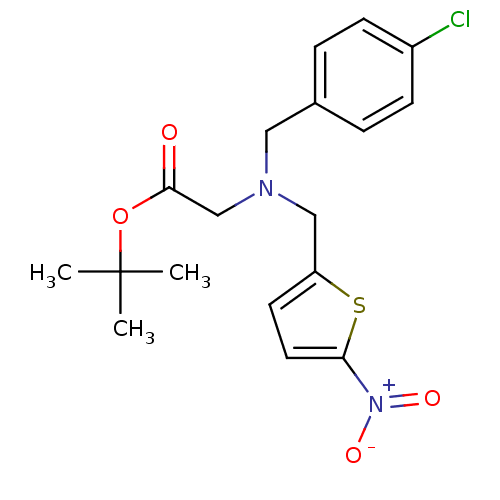
(CHEMBL1961795)Show SMILES CC(C)(C)OC(=O)CN(Cc1ccc(s1)[N+]([O-])=O)Cc1ccc(Cl)cc1 Show InChI InChI=1S/C18H21ClN2O4S/c1-18(2,3)25-17(22)12-20(10-13-4-6-14(19)7-5-13)11-15-8-9-16(26-15)21(23)24/h4-9H,10-12H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50366238
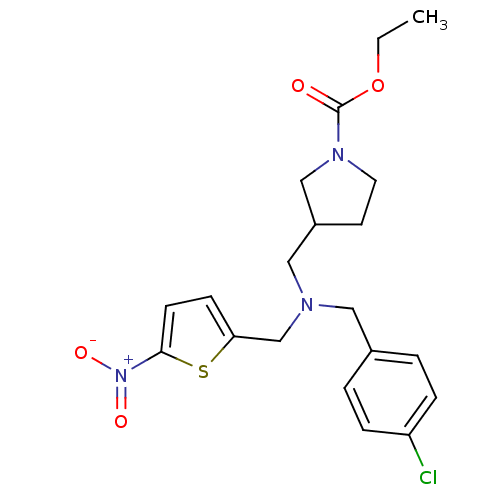
(CHEMBL1961796)Show SMILES CCOC(=O)N1CCC(CN(Cc2ccc(s2)[N+]([O-])=O)Cc2ccc(Cl)cc2)C1 Show InChI InChI=1S/C20H24ClN3O4S/c1-2-28-20(25)23-10-9-16(13-23)12-22(11-15-3-5-17(21)6-4-15)14-18-7-8-19(29-18)24(26)27/h3-8,16H,2,9-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50434044
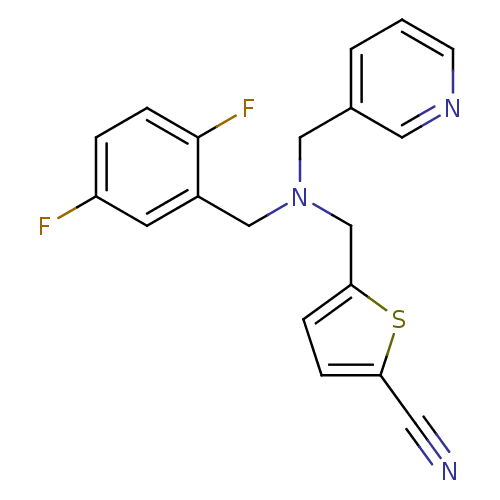
(CHEMBL2381194)Show InChI InChI=1S/C19H15F2N3S/c20-16-3-6-19(21)15(8-16)12-24(11-14-2-1-7-23-10-14)13-18-5-4-17(9-22)25-18/h1-8,10H,11-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50434043
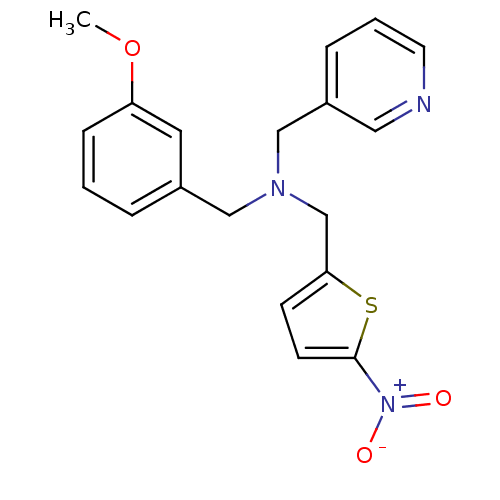
(CHEMBL2381206)Show SMILES COc1cccc(CN(Cc2ccc(s2)[N+]([O-])=O)Cc2cccnc2)c1 Show InChI InChI=1S/C19H19N3O3S/c1-25-17-6-2-4-15(10-17)12-21(13-16-5-3-9-20-11-16)14-18-7-8-19(26-18)22(23)24/h2-11H,12-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50434042
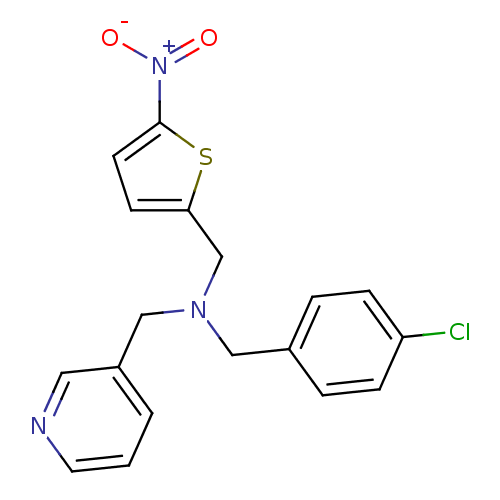
(CHEMBL2381200)Show SMILES [O-][N+](=O)c1ccc(CN(Cc2ccc(Cl)cc2)Cc2cccnc2)s1 Show InChI InChI=1S/C18H16ClN3O2S/c19-16-5-3-14(4-6-16)11-21(12-15-2-1-9-20-10-15)13-17-7-8-18(25-17)22(23)24/h1-10H,11-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50366239
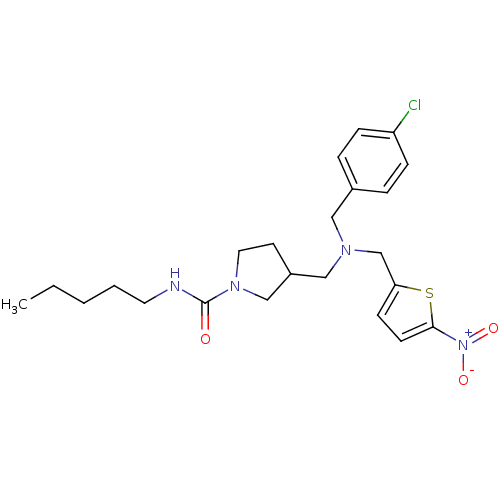
(CHEMBL1961797)Show SMILES CCCCCNC(=O)N1CCC(CN(Cc2ccc(s2)[N+]([O-])=O)Cc2ccc(Cl)cc2)C1 Show InChI InChI=1S/C23H31ClN4O3S/c1-2-3-4-12-25-23(29)27-13-11-19(16-27)15-26(14-18-5-7-20(24)8-6-18)17-21-9-10-22(32-21)28(30)31/h5-10,19H,2-4,11-17H2,1H3,(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50434040
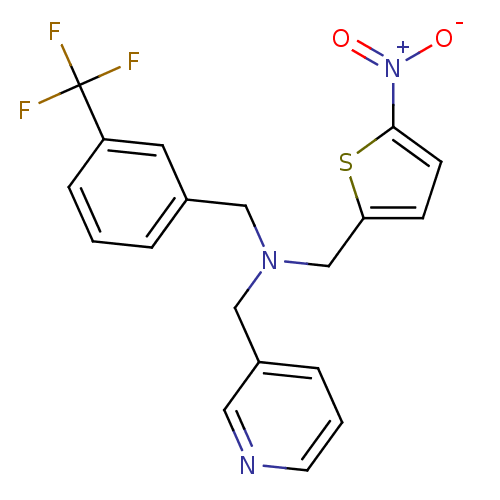
(CHEMBL2381207)Show SMILES [O-][N+](=O)c1ccc(CN(Cc2cccnc2)Cc2cccc(c2)C(F)(F)F)s1 Show InChI InChI=1S/C19H16F3N3O2S/c20-19(21,22)16-5-1-3-14(9-16)11-24(12-15-4-2-8-23-10-15)13-17-6-7-18(28-17)25(26)27/h1-10H,11-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50434039
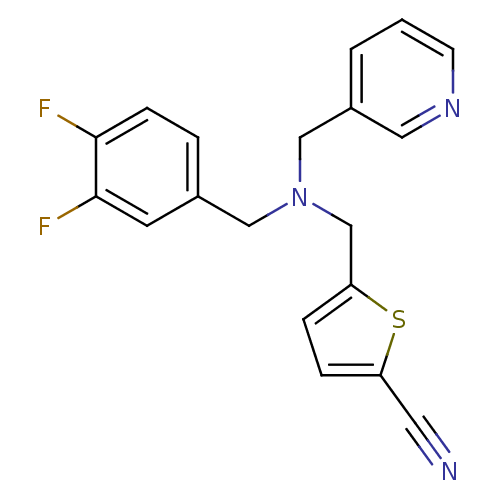
(CHEMBL2381193)Show InChI InChI=1S/C19H15F2N3S/c20-18-6-3-14(8-19(18)21)11-24(12-15-2-1-7-23-10-15)13-17-5-4-16(9-22)25-17/h1-8,10H,11-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50434041
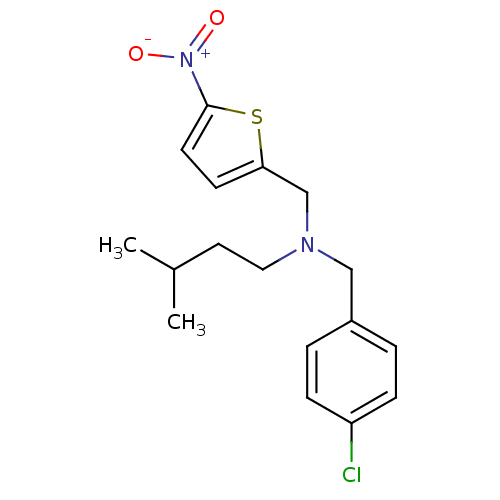
(CHEMBL2381199)Show SMILES CC(C)CCN(Cc1ccc(s1)[N+]([O-])=O)Cc1ccc(Cl)cc1 Show InChI InChI=1S/C17H21ClN2O2S/c1-13(2)9-10-19(11-14-3-5-15(18)6-4-14)12-16-7-8-17(23-16)20(21)22/h3-8,13H,9-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50434038
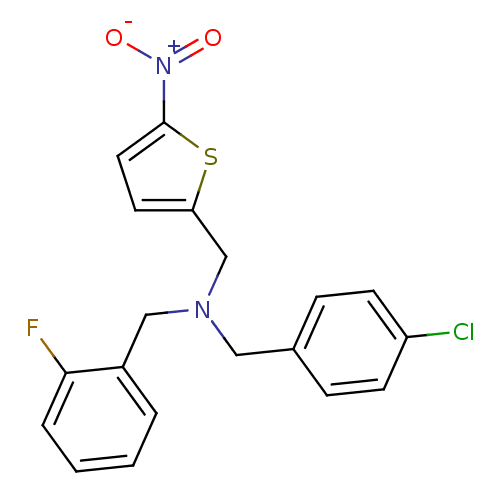
(CHEMBL2381198)Show SMILES [O-][N+](=O)c1ccc(CN(Cc2ccc(Cl)cc2)Cc2ccccc2F)s1 Show InChI InChI=1S/C19H16ClFN2O2S/c20-16-7-5-14(6-8-16)11-22(12-15-3-1-2-4-18(15)21)13-17-9-10-19(26-17)23(24)25/h1-10H,11-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50434037
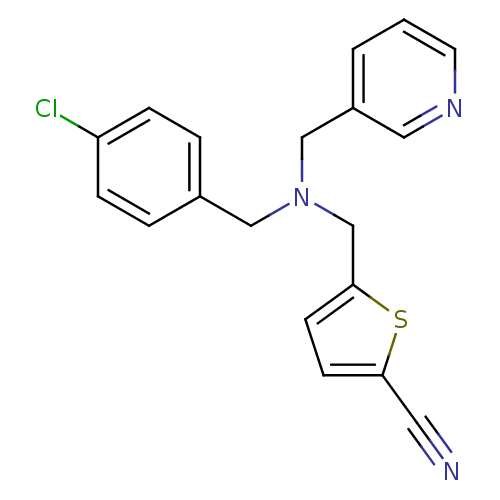
(CHEMBL2381208)Show InChI InChI=1S/C19H16ClN3S/c20-17-5-3-15(4-6-17)12-23(13-16-2-1-9-22-11-16)14-19-8-7-18(10-21)24-19/h1-9,11H,12-14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50434036
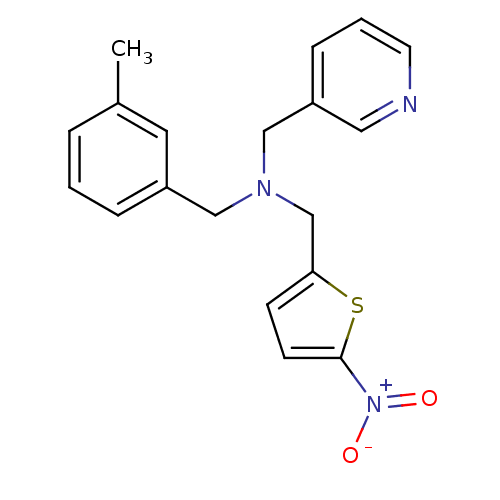
(CHEMBL2381204)Show SMILES Cc1cccc(CN(Cc2ccc(s2)[N+]([O-])=O)Cc2cccnc2)c1 Show InChI InChI=1S/C19H19N3O2S/c1-15-4-2-5-16(10-15)12-21(13-17-6-3-9-20-11-17)14-18-7-8-19(25-18)22(23)24/h2-11H,12-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50434033
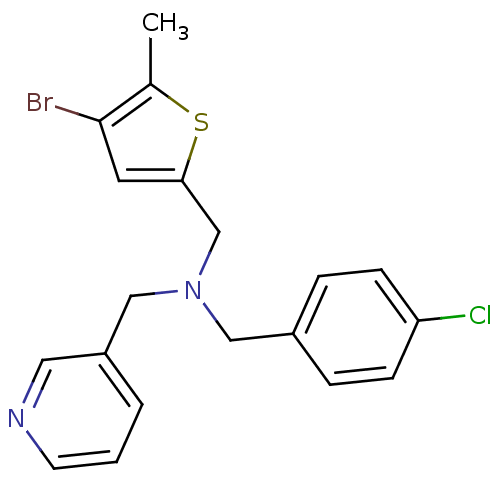
(CHEMBL2381209)Show InChI InChI=1S/C19H18BrClN2S/c1-14-19(20)9-18(24-14)13-23(12-16-3-2-8-22-10-16)11-15-4-6-17(21)7-5-15/h2-10H,11-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50434034
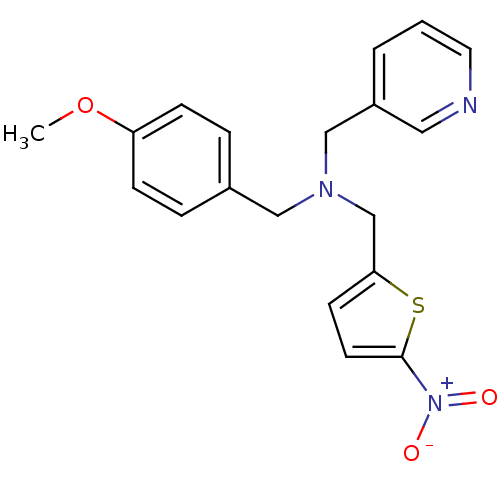
(CHEMBL2381205)Show SMILES COc1ccc(CN(Cc2ccc(s2)[N+]([O-])=O)Cc2cccnc2)cc1 Show InChI InChI=1S/C19H19N3O3S/c1-25-17-6-4-15(5-7-17)12-21(13-16-3-2-10-20-11-16)14-18-8-9-19(26-18)22(23)24/h2-11H,12-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50434032
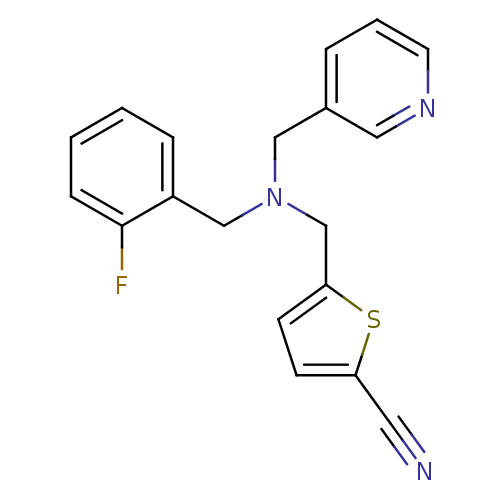
(CHEMBL2381195)Show InChI InChI=1S/C19H16FN3S/c20-19-6-2-1-5-16(19)13-23(12-15-4-3-9-22-11-15)14-18-8-7-17(10-21)24-18/h1-9,11H,12-14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50434035
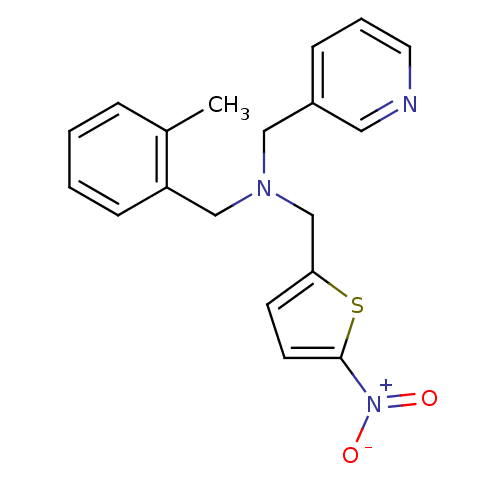
(CHEMBL2381203)Show SMILES Cc1ccccc1CN(Cc1ccc(s1)[N+]([O-])=O)Cc1cccnc1 Show InChI InChI=1S/C19H19N3O2S/c1-15-5-2-3-7-17(15)13-21(12-16-6-4-10-20-11-16)14-18-8-9-19(25-18)22(23)24/h2-11H,12-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50434031
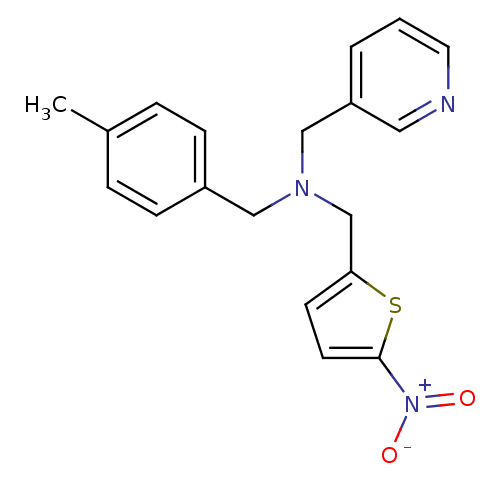
(CHEMBL2381201)Show SMILES Cc1ccc(CN(Cc2ccc(s2)[N+]([O-])=O)Cc2cccnc2)cc1 Show InChI InChI=1S/C19H19N3O2S/c1-15-4-6-16(7-5-15)12-21(13-17-3-2-10-20-11-17)14-18-8-9-19(25-18)22(23)24/h2-11H,12-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50434030
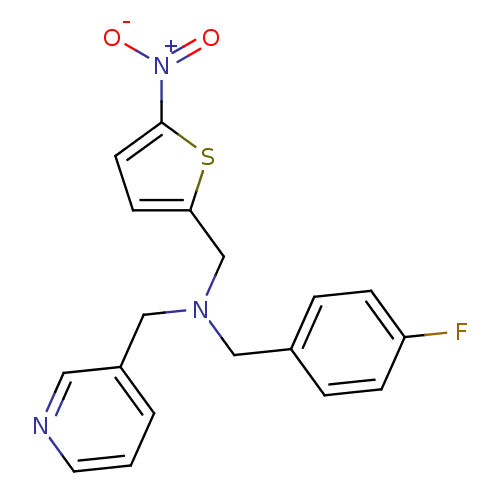
(CHEMBL2381202)Show SMILES [O-][N+](=O)c1ccc(CN(Cc2ccc(F)cc2)Cc2cccnc2)s1 Show InChI InChI=1S/C18H16FN3O2S/c19-16-5-3-14(4-6-16)11-21(12-15-2-1-9-20-10-15)13-17-7-8-18(25-17)22(23)24/h1-10H,11-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
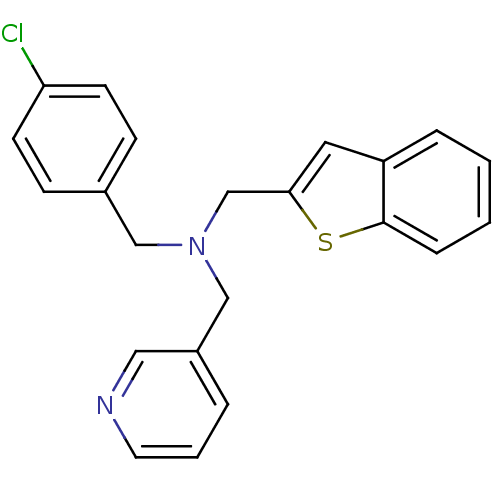
(Homo sapiens (Human)) | BDBM50434029
(CHEMBL2381210)Show InChI InChI=1S/C22H19ClN2S/c23-20-9-7-17(8-10-20)14-25(15-18-4-3-11-24-13-18)16-21-12-19-5-1-2-6-22(19)26-21/h1-13H,14-16H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50434028
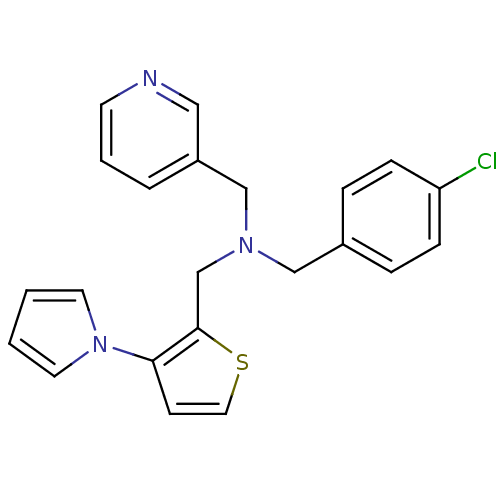
(CHEMBL2381211)Show InChI InChI=1S/C22H20ClN3S/c23-20-7-5-18(6-8-20)15-25(16-19-4-3-10-24-14-19)17-22-21(9-13-27-22)26-11-1-2-12-26/h1-14H,15-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50434027

(CHEMBL2381212)Show InChI InChI=1S/C17H16ClN3S/c18-16-5-3-14(4-6-16)11-21(13-17-20-8-9-22-17)12-15-2-1-7-19-10-15/h1-10H,11-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50434026
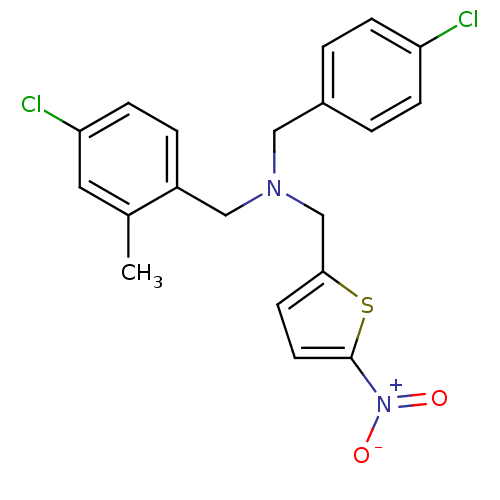
(CHEMBL2381196)Show SMILES Cc1cc(Cl)ccc1CN(Cc1ccc(s1)[N+]([O-])=O)Cc1ccc(Cl)cc1 Show InChI InChI=1S/C20H18Cl2N2O2S/c1-14-10-18(22)7-4-16(14)12-23(11-15-2-5-17(21)6-3-15)13-19-8-9-20(27-19)24(25)26/h2-10H,11-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50434024
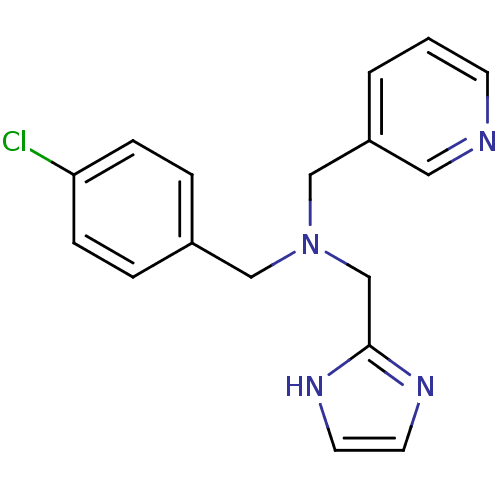
(CHEMBL2381213)Show InChI InChI=1S/C17H17ClN4/c18-16-5-3-14(4-6-16)11-22(13-17-20-8-9-21-17)12-15-2-1-7-19-10-15/h1-10H,11-13H2,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50434025
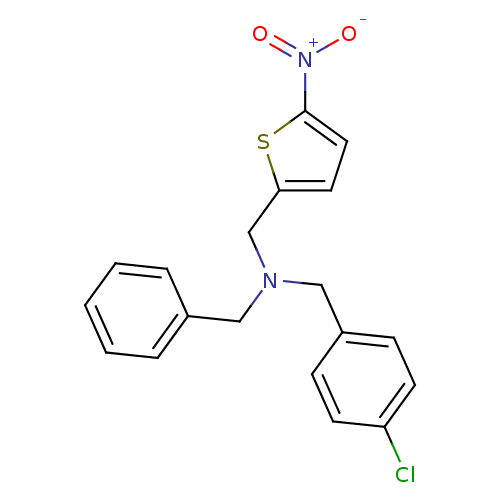
(CHEMBL2381197)Show SMILES [O-][N+](=O)c1ccc(CN(Cc2ccccc2)Cc2ccc(Cl)cc2)s1 Show InChI InChI=1S/C19H17ClN2O2S/c20-17-8-6-16(7-9-17)13-21(12-15-4-2-1-3-5-15)14-18-10-11-19(25-18)22(23)24/h1-11H,12-14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50434023
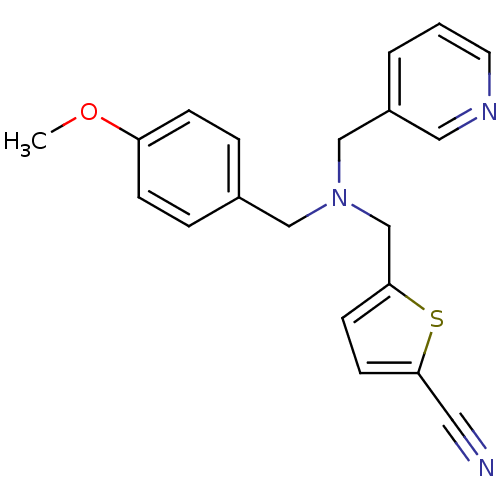
(CHEMBL2380321)Show InChI InChI=1S/C20H19N3OS/c1-24-18-6-4-16(5-7-18)13-23(14-17-3-2-10-22-12-17)15-20-9-8-19(11-21)25-20/h2-10,12H,13-15H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to LXRalpha (unknown origin) by radioligand displacement assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50434033

(CHEMBL2381209)Show InChI InChI=1S/C19H18BrClN2S/c1-14-19(20)9-18(24-14)13-23(12-16-3-2-8-22-10-16)11-15-4-6-17(21)7-5-15/h2-10H,11-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50366239

(CHEMBL1961797)Show SMILES CCCCCNC(=O)N1CCC(CN(Cc2ccc(s2)[N+]([O-])=O)Cc2ccc(Cl)cc2)C1 Show InChI InChI=1S/C23H31ClN4O3S/c1-2-3-4-12-25-23(29)27-13-11-19(16-27)15-26(14-18-5-7-20(24)8-6-18)17-21-9-10-22(32-21)28(30)31/h5-10,19H,2-4,11-17H2,1H3,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50434039

(CHEMBL2381193)Show InChI InChI=1S/C19H15F2N3S/c20-18-6-3-14(8-19(18)21)11-24(12-15-2-1-7-23-10-15)13-17-5-4-16(9-22)25-17/h1-8,10H,11-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50434036

(CHEMBL2381204)Show SMILES Cc1cccc(CN(Cc2ccc(s2)[N+]([O-])=O)Cc2cccnc2)c1 Show InChI InChI=1S/C19H19N3O2S/c1-15-4-2-5-16(10-15)12-21(13-17-6-3-9-20-11-17)14-18-7-8-19(25-18)22(23)24/h2-11H,12-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50434045
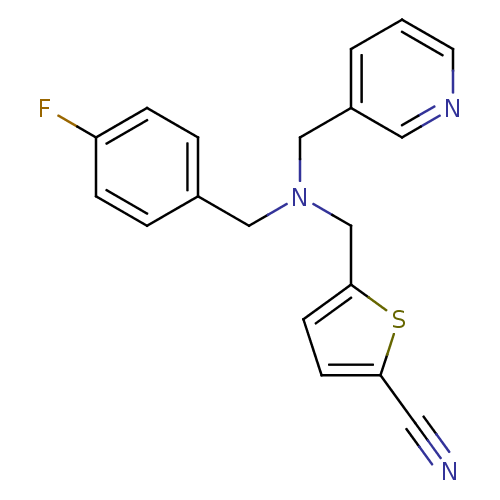
(CHEMBL2381214)Show InChI InChI=1S/C19H16FN3S/c20-17-5-3-15(4-6-17)12-23(13-16-2-1-9-22-11-16)14-19-8-7-18(10-21)24-19/h1-9,11H,12-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50434030

(CHEMBL2381202)Show SMILES [O-][N+](=O)c1ccc(CN(Cc2ccc(F)cc2)Cc2cccnc2)s1 Show InChI InChI=1S/C18H16FN3O2S/c19-16-5-3-14(4-6-16)11-21(12-15-2-1-9-20-10-15)13-17-7-8-18(25-17)22(23)24/h1-10H,11-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50434031

(CHEMBL2381201)Show SMILES Cc1ccc(CN(Cc2ccc(s2)[N+]([O-])=O)Cc2cccnc2)cc1 Show InChI InChI=1S/C19H19N3O2S/c1-15-4-6-16(7-5-15)12-21(13-17-3-2-10-20-11-17)14-18-8-9-19(25-18)22(23)24/h2-11H,12-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50434032

(CHEMBL2381195)Show InChI InChI=1S/C19H16FN3S/c20-19-6-2-1-5-16(19)13-23(12-15-4-3-9-22-11-15)14-18-8-7-17(10-21)24-18/h1-9,11H,12-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50434043

(CHEMBL2381206)Show SMILES COc1cccc(CN(Cc2ccc(s2)[N+]([O-])=O)Cc2cccnc2)c1 Show InChI InChI=1S/C19H19N3O3S/c1-25-17-6-2-4-15(10-17)12-21(13-16-5-3-9-20-11-16)14-18-7-8-19(26-18)22(23)24/h2-11H,12-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50383684

(CHEMBL1961795)Show SMILES CC(C)(C)OC(=O)CN(Cc1ccc(s1)[N+]([O-])=O)Cc1ccc(Cl)cc1 Show InChI InChI=1S/C18H21ClN2O4S/c1-18(2,3)25-17(22)12-20(10-13-4-6-14(19)7-5-13)11-15-8-9-16(26-15)21(23)24/h4-9H,10-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50434023

(CHEMBL2380321)Show InChI InChI=1S/C20H19N3OS/c1-24-18-6-4-16(5-7-18)13-23(14-17-3-2-10-22-12-17)15-20-9-8-19(11-21)25-20/h2-10,12H,13-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 630 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50434034

(CHEMBL2381205)Show SMILES COc1ccc(CN(Cc2ccc(s2)[N+]([O-])=O)Cc2cccnc2)cc1 Show InChI InChI=1S/C19H19N3O3S/c1-25-17-6-4-15(5-7-17)12-21(13-16-3-2-10-20-11-16)14-18-8-9-19(26-18)22(23)24/h2-11H,12-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50434041

(CHEMBL2381199)Show SMILES CC(C)CCN(Cc1ccc(s1)[N+]([O-])=O)Cc1ccc(Cl)cc1 Show InChI InChI=1S/C17H21ClN2O2S/c1-13(2)9-10-19(11-14-3-5-15(18)6-4-14)12-16-7-8-17(23-16)20(21)22/h3-8,13H,9-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50434038

(CHEMBL2381198)Show SMILES [O-][N+](=O)c1ccc(CN(Cc2ccc(Cl)cc2)Cc2ccccc2F)s1 Show InChI InChI=1S/C19H16ClFN2O2S/c20-16-7-5-14(6-8-16)11-22(12-15-3-1-2-4-18(15)21)13-17-9-10-19(26-17)23(24)25/h1-10H,11-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50434037

(CHEMBL2381208)Show InChI InChI=1S/C19H16ClN3S/c20-17-5-3-15(4-6-17)12-23(13-16-2-1-9-22-11-16)14-19-8-7-18(10-21)24-19/h1-9,11H,12-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50434028

(CHEMBL2381211)Show InChI InChI=1S/C22H20ClN3S/c23-20-7-5-18(6-8-20)15-25(16-19-4-3-10-24-14-19)17-22-21(9-13-27-22)26-11-1-2-12-26/h1-14H,15-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 630 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50434040

(CHEMBL2381207)Show SMILES [O-][N+](=O)c1ccc(CN(Cc2cccnc2)Cc2cccc(c2)C(F)(F)F)s1 Show InChI InChI=1S/C19H16F3N3O2S/c20-19(21,22)16-5-1-3-14(9-16)11-24(12-15-4-2-8-23-10-15)13-17-6-7-18(28-17)25(26)27/h1-10H,11-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50434035

(CHEMBL2381203)Show SMILES Cc1ccccc1CN(Cc1ccc(s1)[N+]([O-])=O)Cc1cccnc1 Show InChI InChI=1S/C19H19N3O2S/c1-15-5-2-3-7-17(15)13-21(12-16-6-4-10-20-11-16)14-18-8-9-19(25-18)22(23)24/h2-11H,12-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50366238

(CHEMBL1961796)Show SMILES CCOC(=O)N1CCC(CN(Cc2ccc(s2)[N+]([O-])=O)Cc2ccc(Cl)cc2)C1 Show InChI InChI=1S/C20H24ClN3O4S/c1-2-28-20(25)23-10-9-16(13-23)12-22(11-15-3-5-17(21)6-4-15)14-18-7-8-19(29-18)24(26)27/h3-8,16H,2,9-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50434025

(CHEMBL2381197)Show SMILES [O-][N+](=O)c1ccc(CN(Cc2ccccc2)Cc2ccc(Cl)cc2)s1 Show InChI InChI=1S/C19H17ClN2O2S/c20-17-8-6-16(7-9-17)13-21(12-15-4-2-1-3-5-15)14-18-10-11-19(25-18)22(23)24/h1-11H,12-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50434042

(CHEMBL2381200)Show SMILES [O-][N+](=O)c1ccc(CN(Cc2ccc(Cl)cc2)Cc2cccnc2)s1 Show InChI InChI=1S/C18H16ClN3O2S/c19-16-5-3-14(4-6-16)11-21(12-15-2-1-9-20-10-15)13-17-7-8-18(25-17)22(23)24/h1-10H,11-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50434029

(CHEMBL2381210)Show InChI InChI=1S/C22H19ClN2S/c23-20-9-7-17(8-10-20)14-25(15-18-4-3-11-24-13-18)16-21-12-19-5-1-2-6-22(19)26-21/h1-13H,14-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50434044

(CHEMBL2381194)Show InChI InChI=1S/C19H15F2N3S/c20-16-3-6-19(21)15(8-16)12-24(11-14-2-1-7-23-10-14)13-18-5-4-17(9-22)25-18/h1-8,10H,11-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 630 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50434026

(CHEMBL2381196)Show SMILES Cc1cc(Cl)ccc1CN(Cc1ccc(s1)[N+]([O-])=O)Cc1ccc(Cl)cc1 Show InChI InChI=1S/C20H18Cl2N2O2S/c1-14-10-18(22)7-4-16(14)12-23(11-15-2-5-17(21)6-3-15)13-19-8-9-20(27-19)24(25)26/h2-10H,11-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50434024

(CHEMBL2381213)Show InChI InChI=1S/C17H17ClN4/c18-16-5-3-14(4-6-16)11-22(13-17-20-8-9-21-17)12-15-2-1-7-19-10-15/h1-10H,11-13H2,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group D member 1
(Homo sapiens (Human)) | BDBM50434027

(CHEMBL2381212)Show InChI InChI=1S/C17H16ClN3S/c18-16-5-3-14(4-6-16)11-21(13-17-20-8-9-22-17)12-15-2-1-7-19-10-15/h1-10H,11-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assay |
J Med Chem 56: 4729-37 (2013)
Article DOI: 10.1021/jm400458q
BindingDB Entry DOI: 10.7270/Q20Z74PJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data