 Found 21 hits of Enzyme Inhibition Constant Data
Found 21 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50437704

(CHEMBL2409078)Show InChI InChI=1S/C12H20N2S3/c15-11(13-7-3-1-4-8-13)17-12(16)14-9-5-2-6-10-14/h1-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Horizon Discovery
Curated by ChEMBL
| Assay Description
Competitive inhibition of Pin1 (unknown origin) by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 4283-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.088
BindingDB Entry DOI: 10.7270/Q2DV1M9T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34012
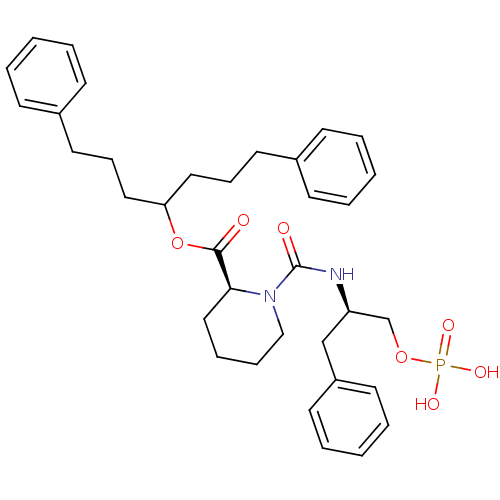
(3-fluorophenylalanine derivative, 21b)Show SMILES OP(O)(=O)OC[C@@H](Cc1cccc(F)c1)NC(=O)c1cc2ccccc2s1 |r| Show InChI InChI=1S/C18H17FNO5PS/c19-14-6-3-4-12(8-14)9-15(11-25-26(22,23)24)20-18(21)17-10-13-5-1-2-7-16(13)27-17/h1-8,10,15H,9,11H2,(H,20,21)(H2,22,23,24)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Horizon Discovery
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 23: 4283-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.088
BindingDB Entry DOI: 10.7270/Q2DV1M9T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50437705

(CHEMBL2409075)Show SMILES Cc1oc(cc1C(=O)N[C@H](Cc1nc2ccccc2[nH]1)C(O)=O)-c1ccc(CN)cc1 |r| Show InChI InChI=1S/C23H22N4O4/c1-13-16(10-20(31-13)15-8-6-14(12-24)7-9-15)22(28)27-19(23(29)30)11-21-25-17-4-2-3-5-18(17)26-21/h2-10,19H,11-12,24H2,1H3,(H,25,26)(H,27,28)(H,29,30)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Horizon Discovery
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 23: 4283-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.088
BindingDB Entry DOI: 10.7270/Q2DV1M9T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34010

(benzothiophene carboxamide, 18b)Show SMILES OP(O)(=O)OC[C@@H](Cc1ccccc1)NC(=O)c1cc2ccccc2s1 |r| Show InChI InChI=1S/C18H18NO5PS/c20-18(17-11-14-8-4-5-9-16(14)26-17)19-15(12-24-25(21,22)23)10-13-6-2-1-3-7-13/h1-9,11,15H,10,12H2,(H,19,20)(H2,21,22,23)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Horizon Discovery
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 23: 4283-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.088
BindingDB Entry DOI: 10.7270/Q2DV1M9T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50437703
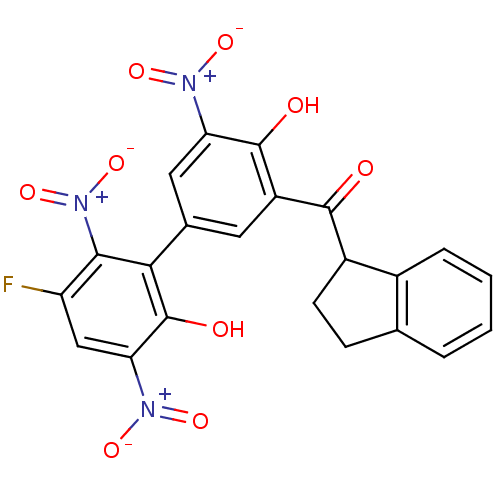
(CHEMBL2409070)Show SMILES Oc1c(cc(cc1[N+]([O-])=O)-c1c(O)c(cc(F)c1[N+]([O-])=O)[N+]([O-])=O)C(=O)C1CCc2ccccc12 Show InChI InChI=1S/C22H14FN3O9/c23-15-9-17(25(32)33)22(29)18(19(15)26(34)35)11-7-14(21(28)16(8-11)24(30)31)20(27)13-6-5-10-3-1-2-4-12(10)13/h1-4,7-9,13,28-29H,5-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Horizon Discovery
Curated by ChEMBL
| Assay Description
Reversible inhibition of human Pin1 |
Bioorg Med Chem Lett 23: 4283-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.088
BindingDB Entry DOI: 10.7270/Q2DV1M9T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50345548

(CHEMBL1784538 | [13C,15N-Y,P,V]cyclic CRYPEVEIC | ...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](-[#8])=O)-[#6](-[#6])-[#6] |r| Show InChI InChI=1S/C47H72N12O15S2/c1-5-24(4)37-44(71)56-32(46(73)74)22-76-75-21-27(48)38(65)52-28(8-6-18-51-47(49)50)39(66)55-31(20-25-10-12-26(60)13-11-25)45(72)59-19-7-9-33(59)42(69)53-29(14-16-34(61)62)40(67)57-36(23(2)3)43(70)54-30(41(68)58-37)15-17-35(63)64/h10-13,23-24,27-33,36-37,60H,5-9,14-22,48H2,1-4H3,(H,52,65)(H,53,69)(H,54,70)(H,55,66)(H,56,71)(H,57,67)(H,58,68)(H,61,62)(H,63,64)(H,73,74)(H4,49,50,51)/t24-,27-,28-,29-,30-,31-,32-,33-,36-,37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Horizon Discovery
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Pin1 (unknown origin) using Suc-AEPF-pNA and WFYpSPR-pNA as substrate after 5 mins |
Bioorg Med Chem Lett 23: 4283-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.088
BindingDB Entry DOI: 10.7270/Q2DV1M9T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50437710
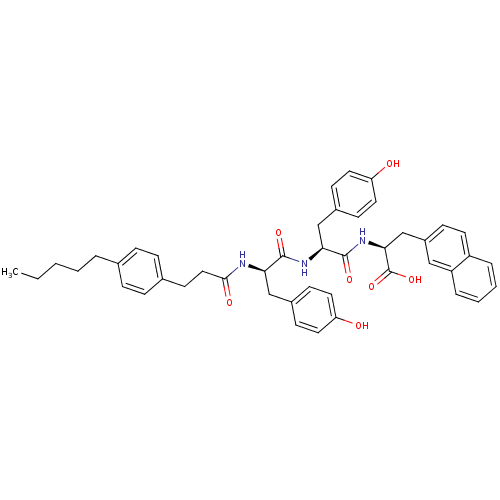
(CHEMBL2409077)Show SMILES CCCCCc1ccc(CCC(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccc3ccccc3c2)C(O)=O)cc1 |r| Show InChI InChI=1S/C45H49N3O7/c1-2-3-4-7-30-10-12-31(13-11-30)19-25-42(51)46-39(27-32-15-21-37(49)22-16-32)43(52)47-40(28-33-17-23-38(50)24-18-33)44(53)48-41(45(54)55)29-34-14-20-35-8-5-6-9-36(35)26-34/h5-6,8-18,20-24,26,39-41,49-50H,2-4,7,19,25,27-29H2,1H3,(H,46,51)(H,47,52)(H,48,53)(H,54,55)/t39-,40+,41+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Horizon Discovery
Curated by ChEMBL
| Assay Description
Inhibition of human Pin1 |
Bioorg Med Chem Lett 23: 4283-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.088
BindingDB Entry DOI: 10.7270/Q2DV1M9T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34005
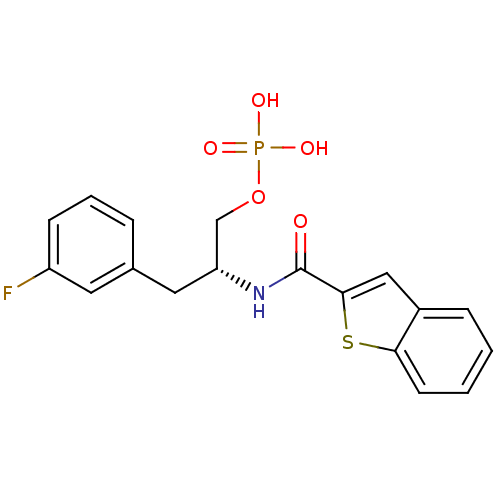
(pipecolate deriv., 12b)Show SMILES OP(O)(=O)OC[C@@H](Cc1ccccc1)NC(=O)N1CCCC[C@H]1C(=O)OC(CCCc1ccccc1)CCCc1ccccc1 |r| Show InChI InChI=1S/C35H45N2O7P/c38-34(44-32(22-12-20-28-14-4-1-5-15-28)23-13-21-29-16-6-2-7-17-29)33-24-10-11-25-37(33)35(39)36-31(27-43-45(40,41)42)26-30-18-8-3-9-19-30/h1-9,14-19,31-33H,10-13,20-27H2,(H,36,39)(H2,40,41,42)/t31-,33+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Horizon Discovery
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 23: 4283-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.088
BindingDB Entry DOI: 10.7270/Q2DV1M9T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50437709
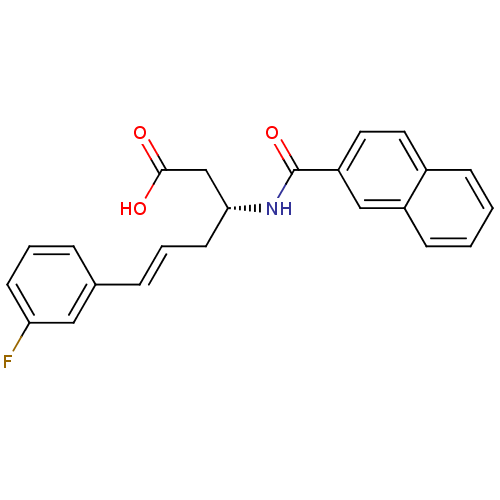
(CHEMBL2409074)Show SMILES OC(=O)C[C@@H](C\C=C\c1cccc(F)c1)NC(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C23H20FNO3/c24-20-9-3-5-16(13-20)6-4-10-21(15-22(26)27)25-23(28)19-12-11-17-7-1-2-8-18(17)14-19/h1-9,11-14,21H,10,15H2,(H,25,28)(H,26,27)/b6-4+/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Horizon Discovery
Curated by ChEMBL
| Assay Description
Inhibition of human Pin1 |
Bioorg Med Chem Lett 23: 4283-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.088
BindingDB Entry DOI: 10.7270/Q2DV1M9T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50437701

(CHEMBL2409067)Show SMILES [#6]-[#6@H](-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@H](-[#6]-[#8][P+]([#8])([#8])[#8-])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-c1ccc(cc1)-[#7+](-[#8-])=O |r| Show InChI InChI=1S/C28H43N10O12P/c1-15(32-17(3)39)23(40)33-16(2)24(41)36-21(14-50-51(47,48)49)27(44)37-13-5-7-22(37)26(43)35-20(6-4-12-31-28(29)30)25(42)34-18-8-10-19(11-9-18)38(45)46/h8-11,15-16,20-22H,4-7,12-14H2,1-3H3,(H,32,39)(H,33,40)(H,34,42)(H,35,43)(H,36,41)(H4,29,30,31)(H2,47,48,49)/t15-,16-,20-,21+,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Horizon Discovery
Curated by ChEMBL
| Assay Description
Reversible inhibition of human Pin1 |
Bioorg Med Chem Lett 23: 4283-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.088
BindingDB Entry DOI: 10.7270/Q2DV1M9T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50437702
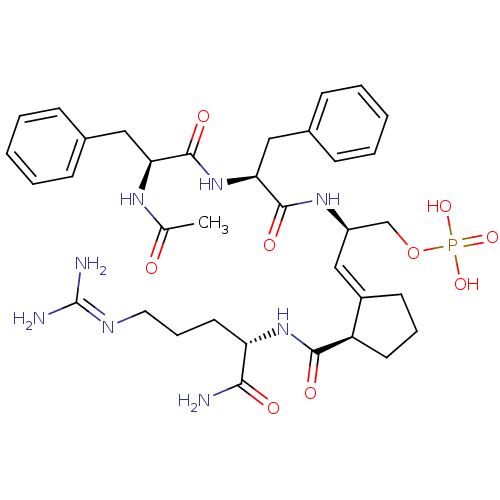
(CHEMBL2409069)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8]P([#8])([#8])=O)\[#6]=[#6]-1\[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C35H49N8O9P/c1-22(44)40-29(18-23-10-4-2-5-11-23)34(48)43-30(19-24-12-6-3-7-13-24)33(47)41-26(21-52-53(49,50)51)20-25-14-8-15-27(25)32(46)42-28(31(36)45)16-9-17-39-35(37)38/h2-7,10-13,20,26-30H,8-9,14-19,21H2,1H3,(H2,36,45)(H,40,44)(H,41,47)(H,42,46)(H,43,48)(H4,37,38,39)(H2,49,50,51)/b25-20-/t26-,27-,28+,29+,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Horizon Discovery
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) using Suc-Ala-Glu-cis-Pro-Phe-pNA as substrate |
Bioorg Med Chem Lett 23: 4283-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.088
BindingDB Entry DOI: 10.7270/Q2DV1M9T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 4
(Homo sapiens (Human)) | BDBM50437711
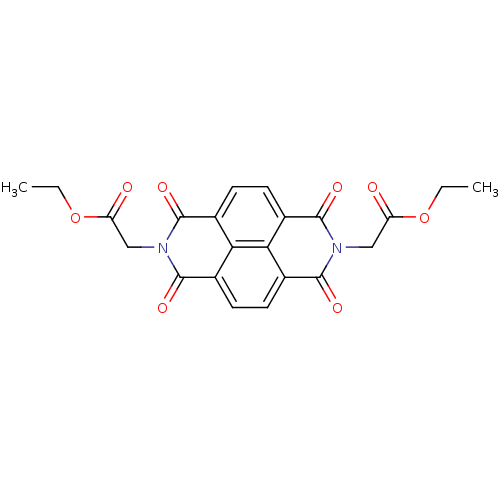
(CHEMBL2409076)Show SMILES CCOC(=O)Cn1c(=O)c2ccc3c4c(ccc(c24)c1=O)c(=O)n(CC(=O)OCC)c3=O Show InChI InChI=1S/C22H18N2O8/c1-3-31-15(25)9-23-19(27)11-5-7-13-18-14(8-6-12(17(11)18)20(23)28)22(30)24(21(13)29)10-16(26)32-4-2/h5-8H,3-4,9-10H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Horizon Discovery
Curated by ChEMBL
| Assay Description
Inhibition of Par14 (unknown origin) |
Bioorg Med Chem Lett 23: 4283-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.088
BindingDB Entry DOI: 10.7270/Q2DV1M9T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50437711

(CHEMBL2409076)Show SMILES CCOC(=O)Cn1c(=O)c2ccc3c4c(ccc(c24)c1=O)c(=O)n(CC(=O)OCC)c3=O Show InChI InChI=1S/C22H18N2O8/c1-3-31-15(25)9-23-19(27)11-5-7-13-18-14(8-6-12(17(11)18)20(23)28)22(30)24(21(13)29)10-16(26)32-4-2/h5-8H,3-4,9-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Horizon Discovery
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) using Suc-Ala-Glu-Pro-Phe-MCA as substrate after 120 secs by fluorescence microtiter plate reader analysis |
Bioorg Med Chem Lett 23: 4283-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.088
BindingDB Entry DOI: 10.7270/Q2DV1M9T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50437708
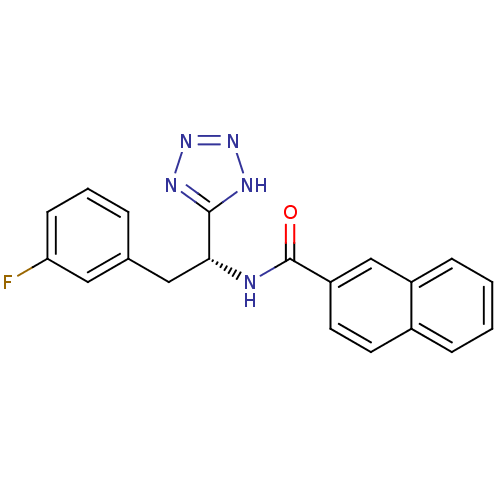
(CHEMBL2409073)Show SMILES Fc1cccc(C[C@@H](NC(=O)c2ccc3ccccc3c2)c2nnn[nH]2)c1 |r| Show InChI InChI=1S/C20H16FN5O/c21-17-7-3-4-13(10-17)11-18(19-23-25-26-24-19)22-20(27)16-9-8-14-5-1-2-6-15(14)12-16/h1-10,12,18H,11H2,(H,22,27)(H,23,24,25,26)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Horizon Discovery
Curated by ChEMBL
| Assay Description
Inhibition of human Pin1 |
Bioorg Med Chem Lett 23: 4283-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.088
BindingDB Entry DOI: 10.7270/Q2DV1M9T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50437704

(CHEMBL2409078)Show InChI InChI=1S/C12H20N2S3/c15-11(13-7-3-1-4-8-13)17-12(16)14-9-5-2-6-10-14/h1-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Horizon Discovery
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 23: 4283-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.088
BindingDB Entry DOI: 10.7270/Q2DV1M9T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50437706

(CHEMBL2409071)Show InChI InChI=1S/C18H17FO2/c1-18(10-9-12-5-3-4-6-15(12)18)17(20)14-11-13(19)7-8-16(14)21-2/h3-8,11H,9-10H2,1-2H3/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Horizon Discovery
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 23: 4283-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.088
BindingDB Entry DOI: 10.7270/Q2DV1M9T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50437707
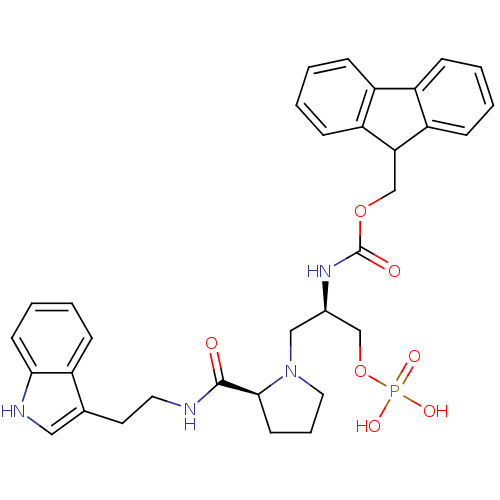
(CHEMBL2409072)Show SMILES OP(O)(=O)OC[C@@H](CN1CCC[C@H]1C(=O)NCCc1c[nH]c2ccccc12)NC(=O)OCC1c2ccccc2-c2ccccc12 |r| Show InChI InChI=1S/C33H37N4O7P/c38-32(34-16-15-22-18-35-30-13-6-5-8-24(22)30)31-14-7-17-37(31)19-23(20-44-45(40,41)42)36-33(39)43-21-29-27-11-3-1-9-25(27)26-10-2-4-12-28(26)29/h1-6,8-13,18,23,29,31,35H,7,14-17,19-21H2,(H,34,38)(H,36,39)(H2,40,41,42)/t23-,31+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Horizon Discovery
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 23: 4283-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.088
BindingDB Entry DOI: 10.7270/Q2DV1M9T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50247868
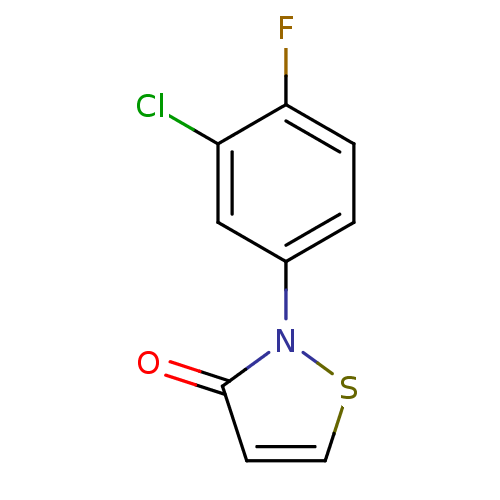
(2-(3-Chloro-4-fluorophenyl)isothiazol-3(2H)-one | ...)Show InChI InChI=1S/C9H5ClFNOS/c10-7-5-6(1-2-8(7)11)12-9(13)3-4-14-12/h1-5H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Horizon Discovery
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged Pin1 (unknown origin) by SPR spectroscopic analysis |
Bioorg Med Chem Lett 23: 4283-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.088
BindingDB Entry DOI: 10.7270/Q2DV1M9T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50314721
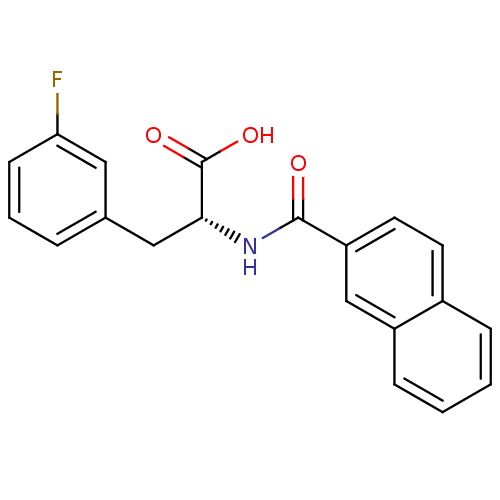
((R)-2-(2-naphthamido)-3-(3-fluorophenyl)propanoic ...)Show SMILES OC(=O)[C@@H](Cc1cccc(F)c1)NC(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C20H16FNO3/c21-17-7-3-4-13(10-17)11-18(20(24)25)22-19(23)16-9-8-14-5-1-2-6-15(14)12-16/h1-10,12,18H,11H2,(H,22,23)(H,24,25)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Horizon Discovery
Curated by ChEMBL
| Assay Description
Inhibition of human Pin1 |
Bioorg Med Chem Lett 23: 4283-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.088
BindingDB Entry DOI: 10.7270/Q2DV1M9T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50070942

((-)-Epigallocatechin gallate | (-)-Epigallocatechi...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Horizon Discovery
Curated by ChEMBL
| Assay Description
Inhibition of full length Pin1 (unknown origin) using WFY(pS)PR-pNA as substrate by spectrophotometric analysis |
Bioorg Med Chem Lett 23: 4283-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.088
BindingDB Entry DOI: 10.7270/Q2DV1M9T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170737
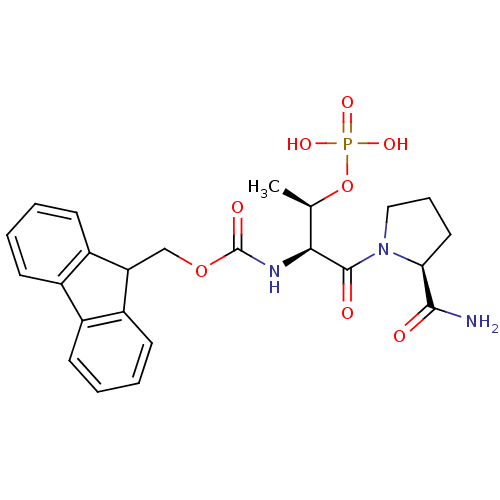
(CHEMBL360409 | [(S)-1-((S)-2-Carbamoyl-pyrrolidine...)Show SMILES C[C@@H](OP(O)(O)=O)[C@H](NC(=O)OCC1c2ccccc2-c2ccccc12)C(=O)N1CCC[C@H]1C(N)=O Show InChI InChI=1S/C24H28N3O8P/c1-14(35-36(31,32)33)21(23(29)27-12-6-11-20(27)22(25)28)26-24(30)34-13-19-17-9-4-2-7-15(17)16-8-3-5-10-18(16)19/h2-5,7-10,14,19-21H,6,11-13H2,1H3,(H2,25,28)(H,26,30)(H2,31,32,33)/t14-,20+,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a |
Horizon Discovery
Curated by ChEMBL
| Assay Description
Binding affinity to Pin1 WW domain (unknown origin) |
Bioorg Med Chem Lett 23: 4283-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.088
BindingDB Entry DOI: 10.7270/Q2DV1M9T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data