

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM50442322 (CHEMBL2442760) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM13061 (4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442326 (CHEMBL2442758) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442323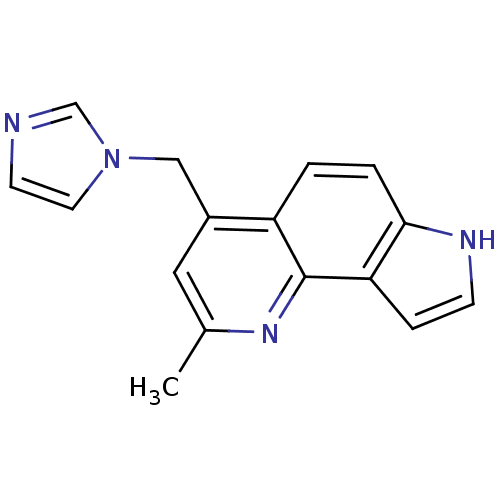 (CHEMBL2442759) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442325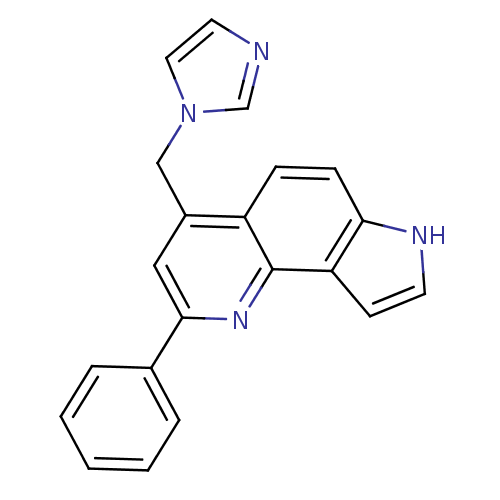 (CHEMBL2442756) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442320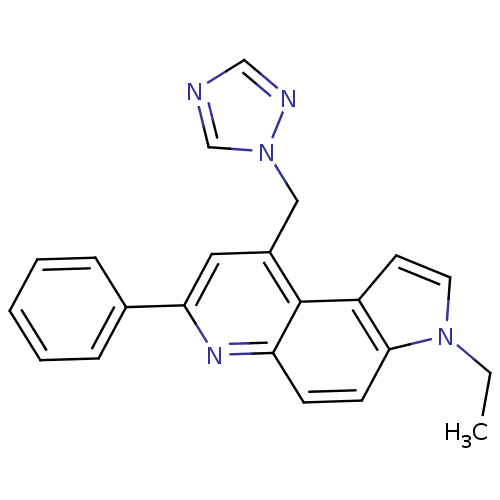 (CHEMBL2442762) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50442323 (CHEMBL2442759) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50442325 (CHEMBL2442756) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50442326 (CHEMBL2442758) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442324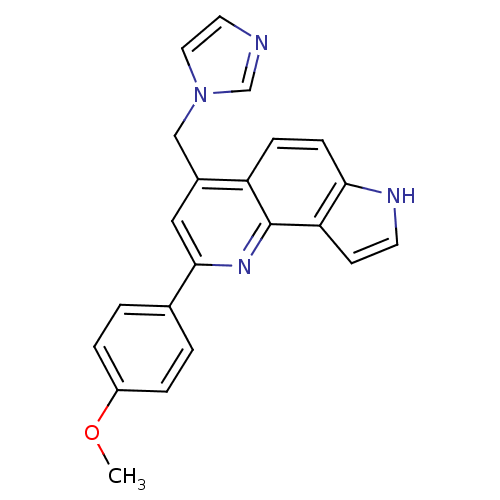 (CHEMBL2442757) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 454 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50442326 (CHEMBL2442758) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using progesterone as substrate NADPH as cofactor | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50442324 (CHEMBL2442757) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using progesterone as substrate NADPH as cofactor | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442316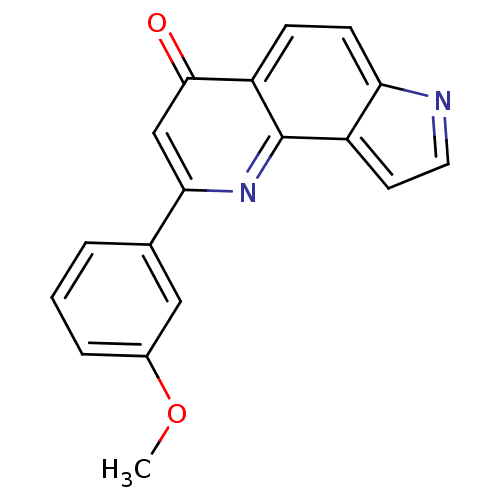 (CHEMBL2442766) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50442325 (CHEMBL2442756) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using progesterone as substrate NADPH as cofactor | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442330 (CHEMBL2440145) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 922 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50442324 (CHEMBL2442757) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50442322 (CHEMBL2442760) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442317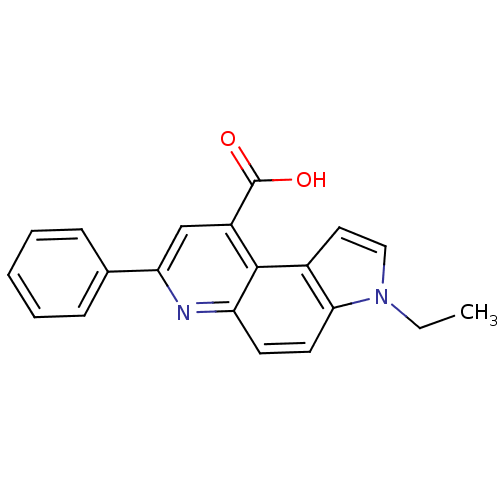 (CHEMBL2442765) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50442320 (CHEMBL2442762) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442331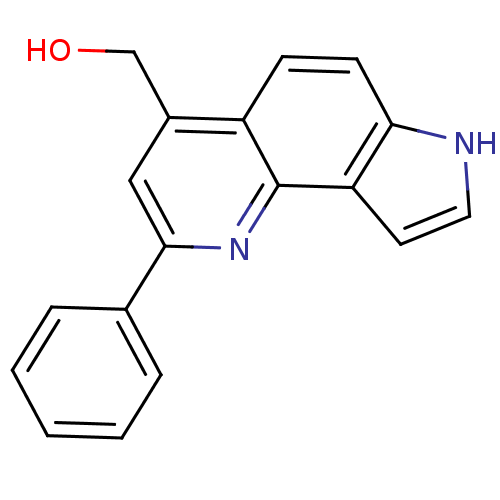 (CHEMBL2442772) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50442322 (CHEMBL2442760) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using progesterone as substrate NADPH as cofactor | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442328 (CHEMBL2442754) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50442321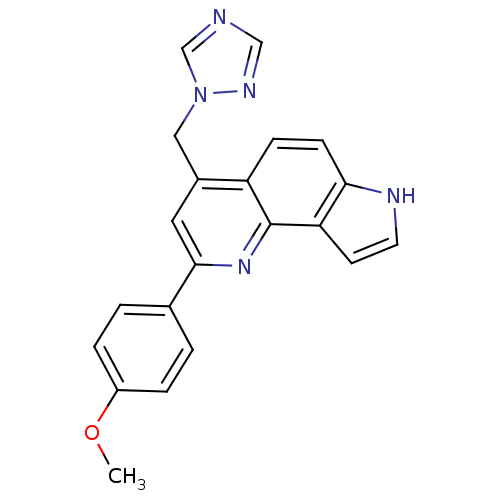 (CHEMBL2442761) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using progesterone as substrate NADPH as cofactor | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50442320 (CHEMBL2442762) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using progesterone as substrate NADPH as cofactor | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50442321 (CHEMBL2442761) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50442323 (CHEMBL2442759) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using progesterone as substrate NADPH as cofactor | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442318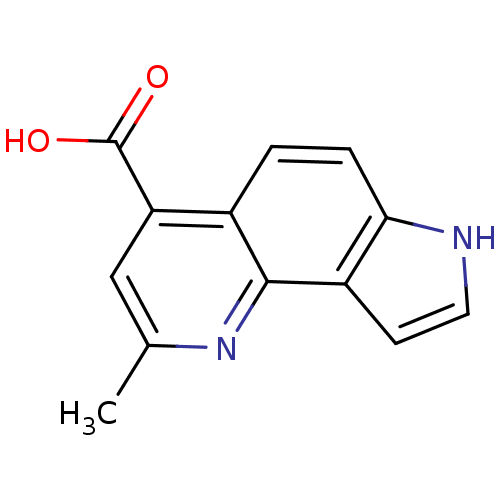 (CHEMBL2442764) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442329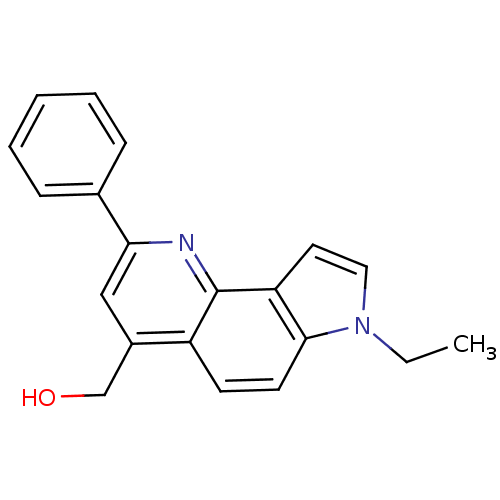 (CHEMBL2442753) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442321 (CHEMBL2442761) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442335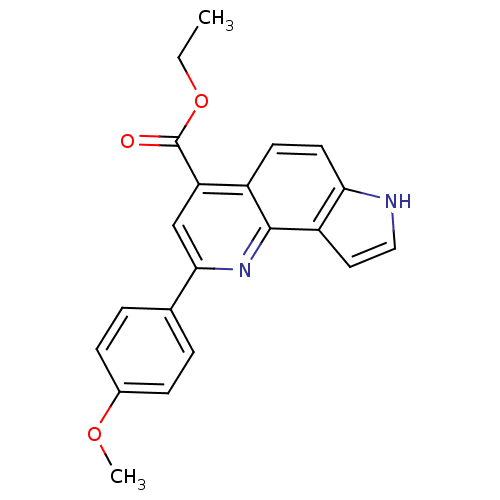 (CHEMBL2442768) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442336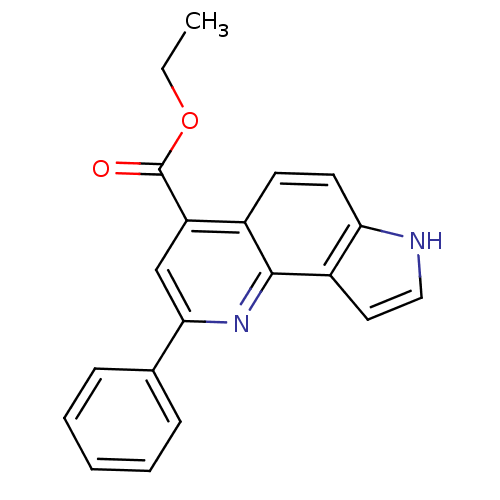 (CHEMBL2442767) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442332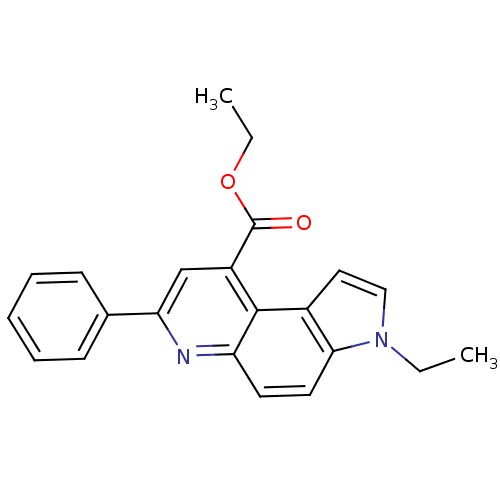 (CHEMBL2442771) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442319 (CHEMBL2442763) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442334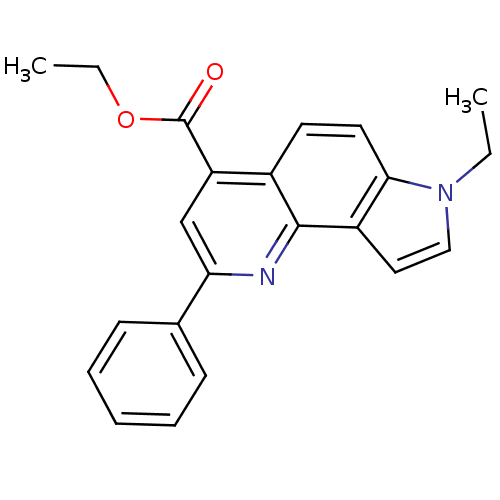 (CHEMBL2442769) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442333 (CHEMBL2442770) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50442327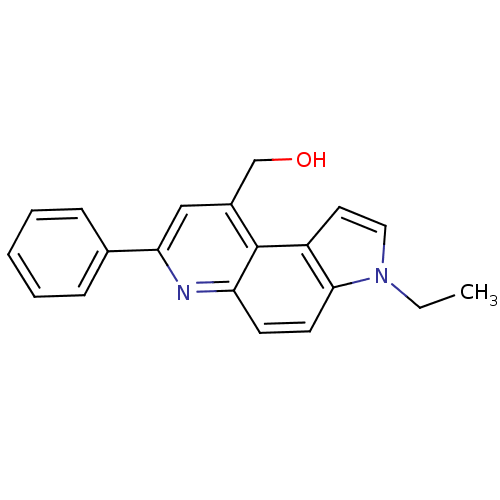 (CHEMBL2442755) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis | J Med Chem 56: 7536-51 (2013) Article DOI: 10.1021/jm400377z BindingDB Entry DOI: 10.7270/Q2M046WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||