

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50019160 (CHEMBL3289100) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of microsomal PGES1 isolated from IL-1beta-stimulated human A549 cells preincubated for 15 mins followed by substrate addition measured af... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50019157 (CHEMBL3289097) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of microsomal PGES1 isolated from IL-1beta-stimulated human A549 cells preincubated for 15 mins followed by substrate addition measured af... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50303448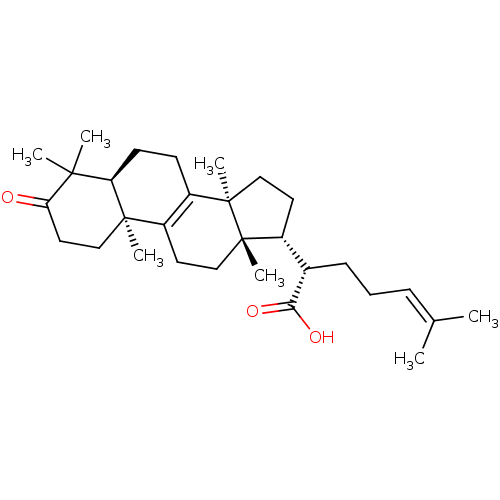 (Beta-elemonic acid | CHEMBL566929) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of microsomal PGES1 isolated from IL-1beta-stimulated human A549 cells preincubated for 15 mins followed by substrate addition measured af... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50019154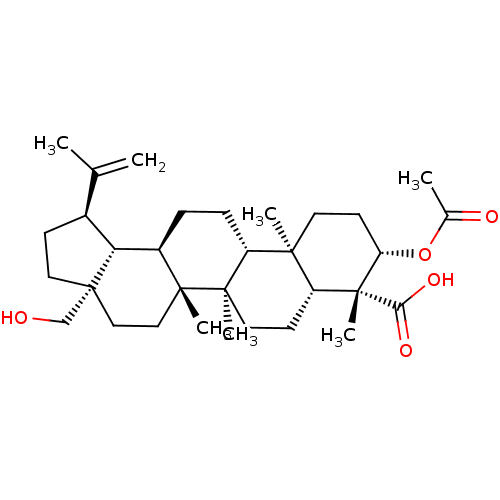 (CHEMBL3289107) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of microsomal PGES1 isolated from IL-1beta-stimulated human A549 cells preincubated for 15 mins followed by substrate addition measured af... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50019162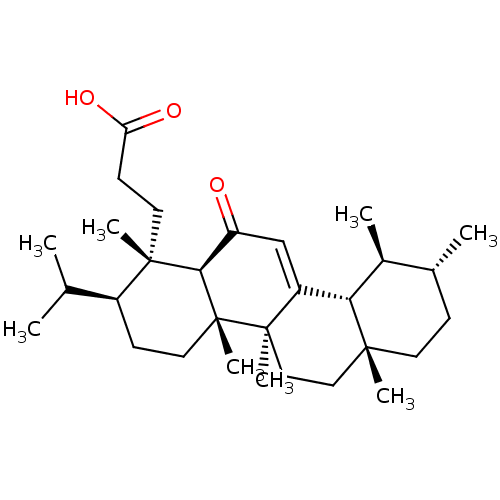 (CHEMBL3289103) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of microsomal PGES1 isolated from IL-1beta-stimulated human A549 cells preincubated for 15 mins followed by substrate addition measured af... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50019156 (CHEBI:10274 | CHEMBL3289096) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of microsomal PGES1 isolated from IL-1beta-stimulated human A549 cells preincubated for 15 mins followed by substrate addition measured af... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50019158 (CHEMBL3289098) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of microsomal PGES1 isolated from IL-1beta-stimulated human A549 cells preincubated for 15 mins followed by substrate addition measured af... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50006805 (3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of microsomal PGES1 isolated from IL-1beta-stimulated human A549 cells preincubated for 15 mins followed by substrate addition measured af... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50241261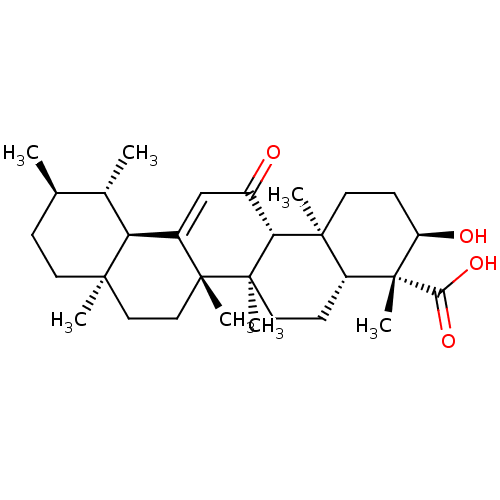 ((3R,4R,4aR,6aR,6bS,8aR,11R,12S,12aR,14aR,14bS)-3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of microsomal PGES1 isolated from IL-1beta-stimulated human A549 cells preincubated for 15 mins followed by substrate addition measured af... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50019163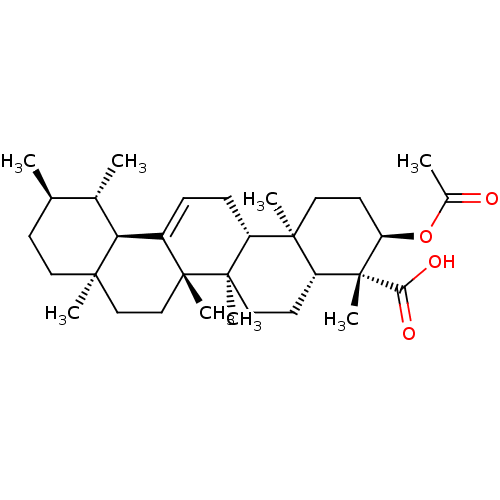 (Acetyl-Beta-Boswellic Acid | CHEMBL236906) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of microsomal PGES1 isolated from IL-1beta-stimulated human A549 cells preincubated for 15 mins followed by substrate addition measured af... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50019159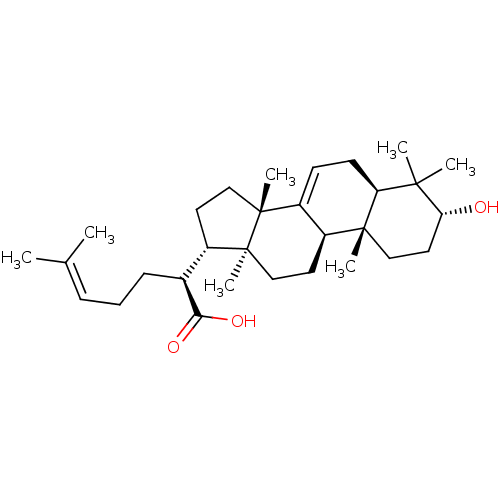 (CHEMBL3289099) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of microsomal PGES1 isolated from IL-1beta-stimulated human A549 cells preincubated for 15 mins followed by substrate addition measured af... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50241262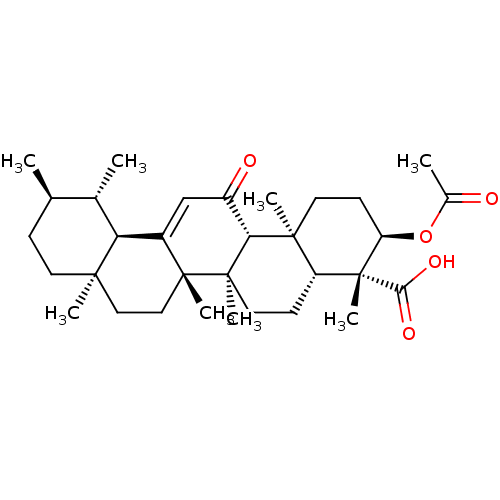 ((3R,4R,4aR,6aR,6bS,8aR,11R,12S,12aR,14aR,14bS)-3-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of microsomal PGES1 isolated from IL-1beta-stimulated human A549 cells preincubated for 15 mins followed by substrate addition measured af... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50241260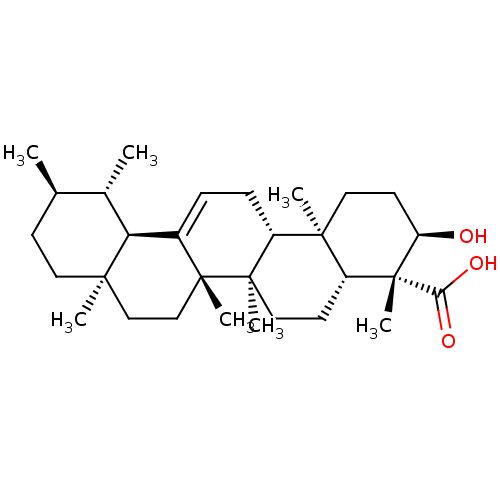 ((3R,4R,4aR,6aR,6bS,8aR,11R,12S,12aR,14aR,14bR)-3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of microsomal PGES1 isolated from IL-1beta-stimulated human A549 cells preincubated for 15 mins followed by substrate addition measured af... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50019152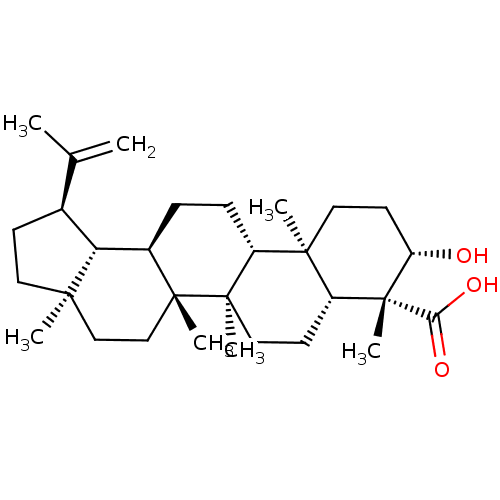 (CHEMBL3289105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of microsomal PGES1 isolated from IL-1beta-stimulated human A549 cells preincubated for 15 mins followed by substrate addition measured af... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50019155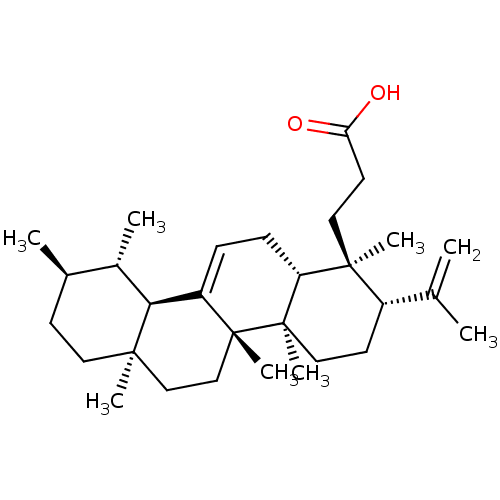 (CHEMBL3289101) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 mins by HPLC an... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50019155 (CHEMBL3289101) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 using arachidonic acid as substrate assessed as PGE2 formation after 2 mins by LC-MS/MS analysis | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50019161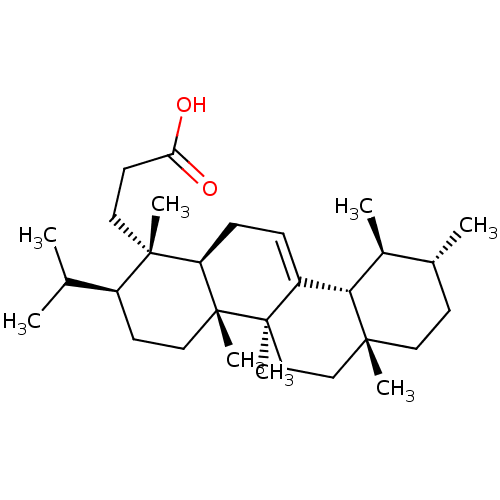 (CHEMBL3289102) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 min... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50019161 (CHEMBL3289102) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of microsomal PGES1 isolated from IL-1beta-stimulated human A549 cells preincubated for 15 mins followed by substrate addition measured af... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50019157 (CHEMBL3289097) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 mins by HPLC an... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50019159 (CHEMBL3289099) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 mins by HPLC an... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50019152 (CHEMBL3289105) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 mins by HPLC an... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50019153 (CHEMBL3289106) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of microsomal PGES1 isolated from IL-1beta-stimulated human A549 cells preincubated for 15 mins followed by substrate addition measured af... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50019155 (CHEMBL3289101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of human COX-2 using arachidonic acid as substrate assessed as PGE2 formation after 2 mins by LC-MS/MS analysis | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50303448 (Beta-elemonic acid | CHEMBL566929) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 mins by HPLC an... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50019151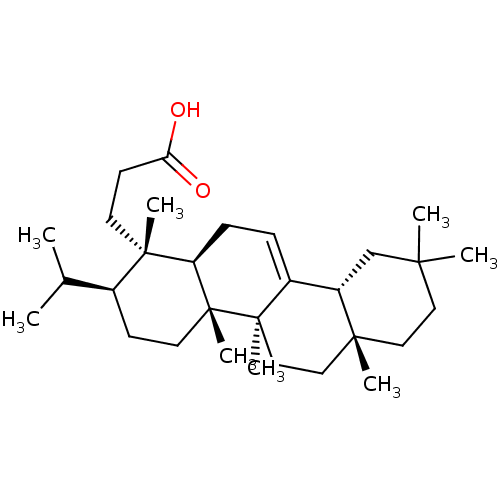 (CHEMBL3289104) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 mins by HPLC an... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50019153 (CHEMBL3289106) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 mins by HPLC an... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50019155 (CHEMBL3289101) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of microsomal PGES1 isolated from IL-1beta-stimulated human A549 cells preincubated for 15 mins followed by substrate addition measured af... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50019156 (CHEBI:10274 | CHEMBL3289096) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 mins by HPLC an... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50019157 (CHEMBL3289097) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 min... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50303448 (Beta-elemonic acid | CHEMBL566929) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 min... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50019155 (CHEMBL3289101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 min... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50019154 (CHEMBL3289107) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 min... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50019161 (CHEMBL3289102) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 mins by HPLC an... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50019160 (CHEMBL3289100) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 mins by HPLC an... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50019152 (CHEMBL3289105) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 min... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50019151 (CHEMBL3289104) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 min... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50019159 (CHEMBL3289099) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 min... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50019162 (CHEMBL3289103) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 mins by HPLC an... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50019151 (CHEMBL3289104) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of microsomal PGES1 isolated from IL-1beta-stimulated human A549 cells preincubated for 15 mins followed by substrate addition measured af... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50019153 (CHEMBL3289106) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 min... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50019160 (CHEMBL3289100) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 min... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50019158 (CHEMBL3289098) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 min... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50019156 (CHEBI:10274 | CHEMBL3289096) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 min... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50019162 (CHEMBL3289103) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 min... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50019158 (CHEMBL3289098) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 mins by HPLC an... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50019154 (CHEMBL3289107) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 mins by HPLC an... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||