

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.41E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152478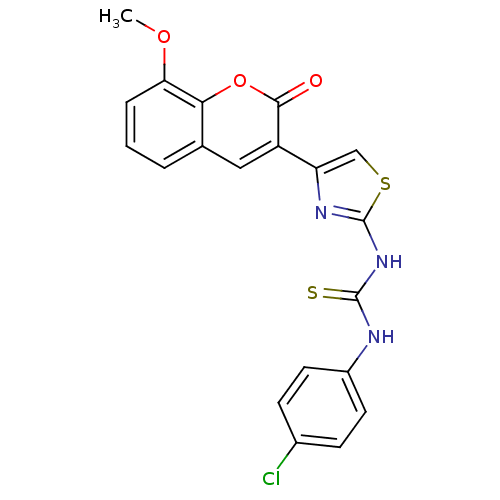 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.58E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152467 (1-(4-Fluorophenyl)-3-(4-(6-nitro-2-oxo-2H-chromen-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.93E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152472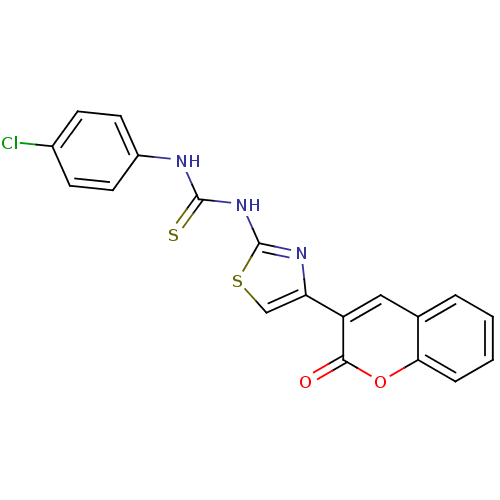 (1-(4-Chlorophenyl)-3-(4-(2-oxo-2H-chromen-3-yl)thi...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.63E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152478 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152471 (1-(3-Fluorophenyl)-3-(4-(2-oxo-2H-chromen-3-yl)thi...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152476 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152476 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.84E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152471 (1-(3-Fluorophenyl)-3-(4-(2-oxo-2H-chromen-3-yl)thi...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.08E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152475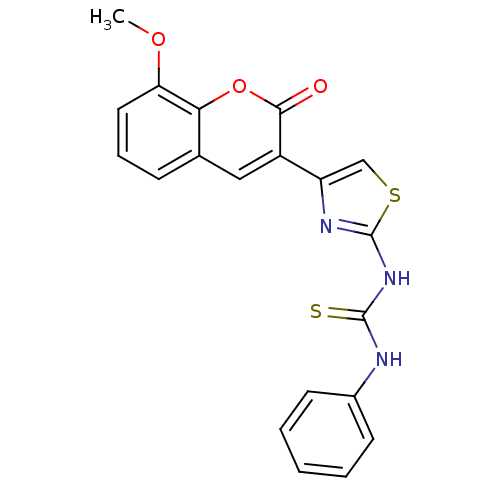 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.18E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152477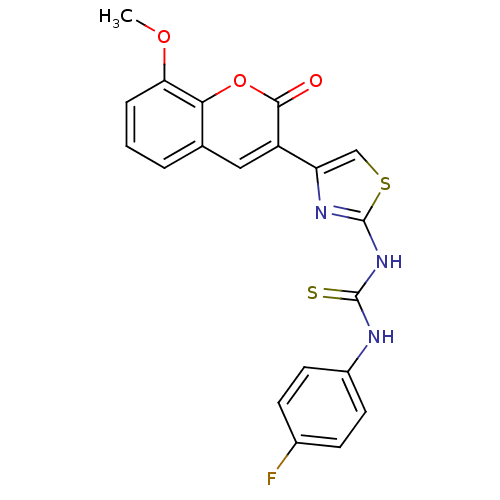 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.33E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152472 (1-(4-Chlorophenyl)-3-(4-(2-oxo-2H-chromen-3-yl)thi...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.43E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152470 (1-(4-Iodophenyl)-3-(4-(6-nitro-2-oxo-2H-chromen-3-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.67E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152437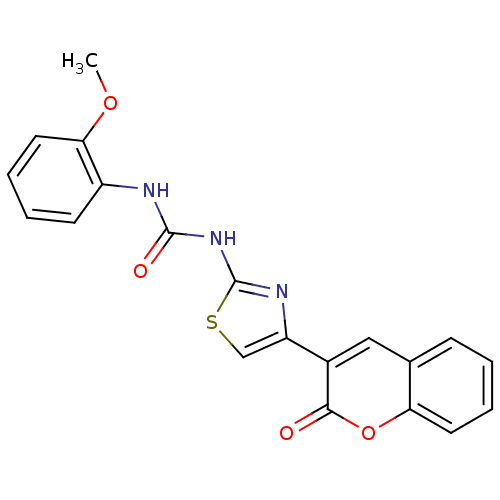 (1-(2-Methoxyphenyl)-3-(4-(2-oxo-2H-chromen-3-yl)th...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.03E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152473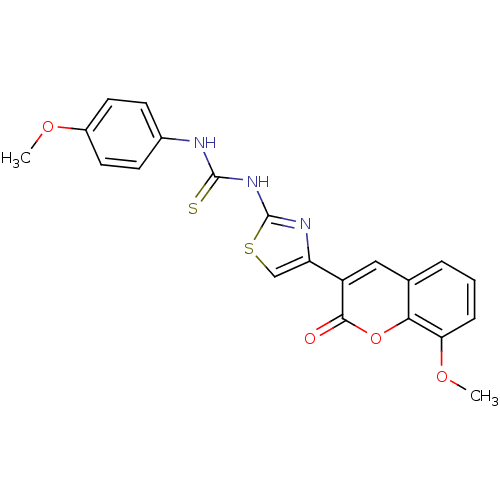 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.12E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152443 (1-(2-Flurophenyl)-3-(4-(2-oxo-2H-chromen-3-yl)thia...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.15E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152443 (1-(2-Flurophenyl)-3-(4-(2-oxo-2H-chromen-3-yl)thia...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.45E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152474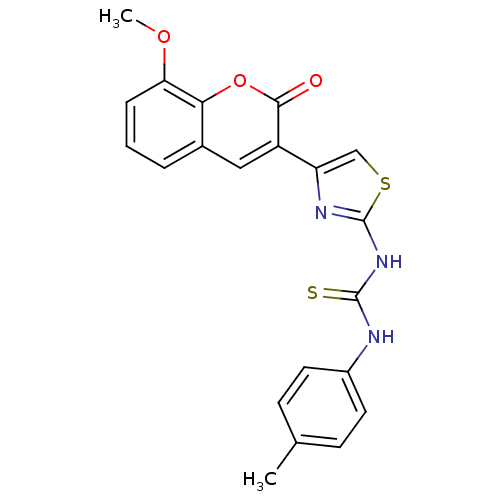 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.65E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152437 (1-(2-Methoxyphenyl)-3-(4-(2-oxo-2H-chromen-3-yl)th...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152447 (1-(4-Chlorophenyl)-3-(4-(2-oxo-2H-chromen-3-yl)thi...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.81E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152463 (1-(3-Methoxyphenyl)-3-(4-(6-nitro-2-oxo-2H-chromen...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.87E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152473 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.12E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152475 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.61E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152477 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.61E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152469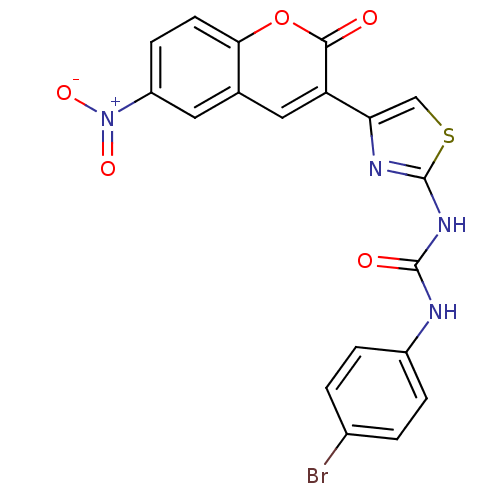 (1-(4-Bromophenyl)-3-(4-(6-nitro-2-oxo-2H-chromen-3...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.62E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152455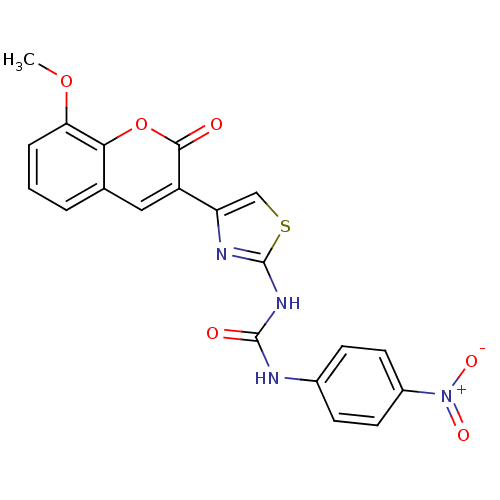 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.65E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152474 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.19E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152442 (1-(4-Nitrophenyl)-3-(4-(2-oxo-2H-chromen-3-yl)thia...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.27E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152438 (1-(3-Methoxyphenyl)-3-(4-(2-oxo-2H-chromen-3-yl)th...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.28E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152440 (1-(p-tolyl)-3-(4-(2-oxo-2H-chromen-3-yl)thiazol-2-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.48E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152450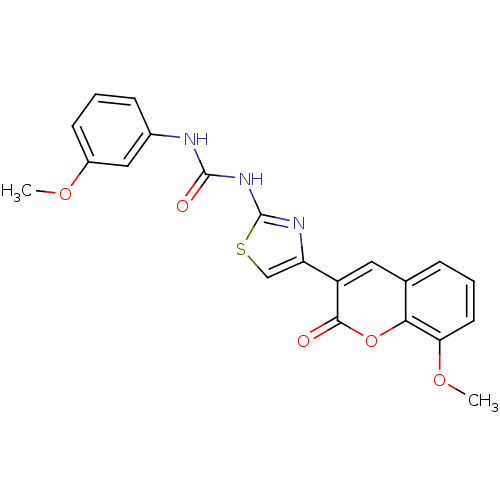 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.65E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152444 (1-(3-Fluorophenyl)-3-(4-(2-oxo-2H-chromen-3-yl)thi...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.81E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152462 (1-(4-Iodophenyl)-3-(4-(8-methoxy-2-oxo-2H-chromen-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.13E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152444 (1-(3-Fluorophenyl)-3-(4-(2-oxo-2H-chromen-3-yl)thi...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.22E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152459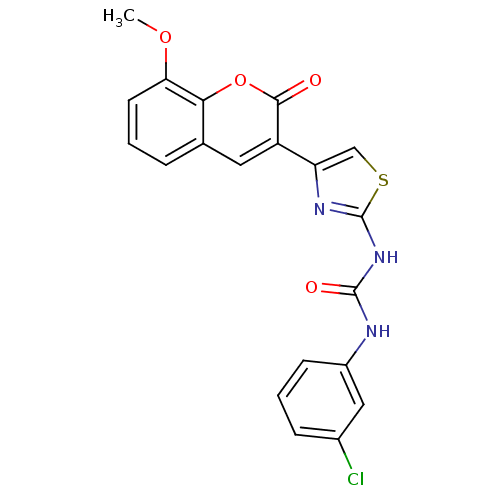 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.29E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152458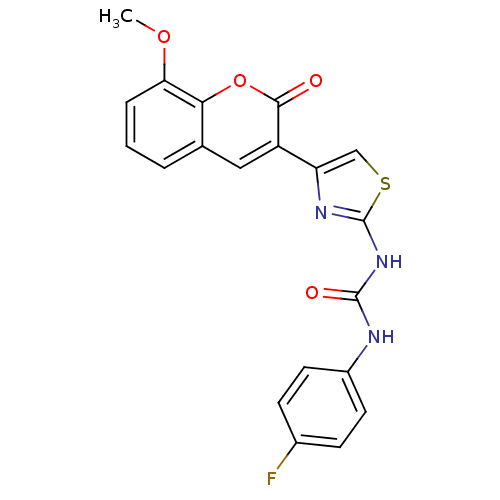 (1-(4-Fluorophenyl)-3-(4-(8-methoxy-2-oxo-2H-chrome...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.33E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152456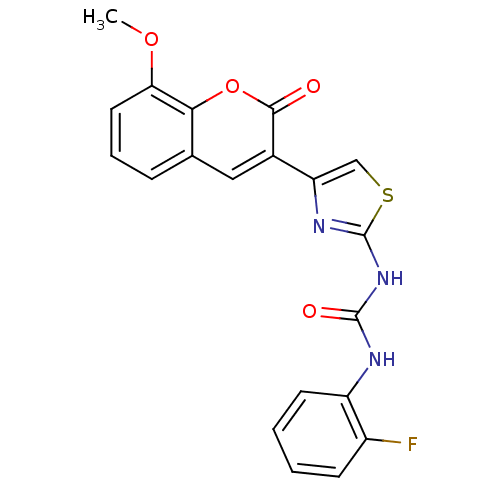 (1-(2-Fluorophenyl)-3-(4-(8-methoxy-2-oxo-2H-chrome...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.62E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152446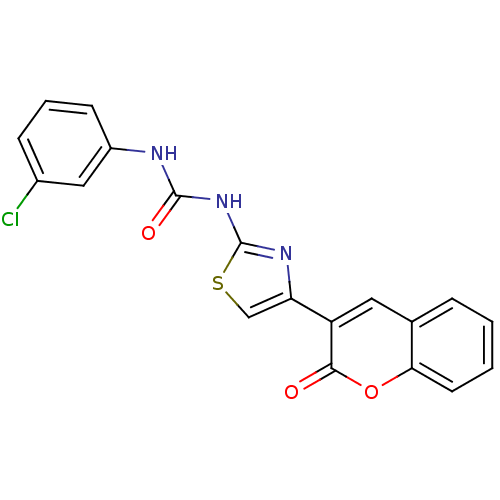 (1-(3-Chlorophenyl)-3-(4-(2-oxo-2H-chromen-3-yl)thi...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.66E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152457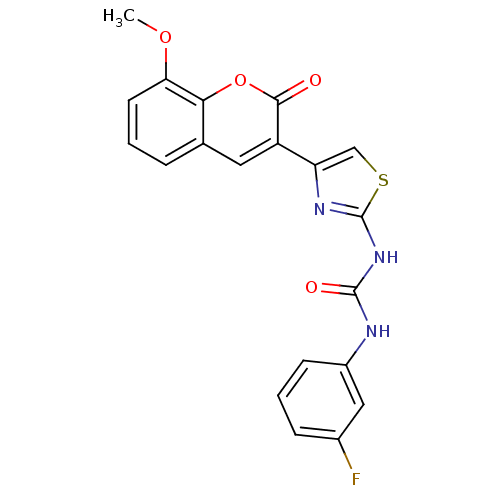 (1-(3-Fluorophenyl)-3-(4-(8-methoxy-2-oxo-2H-chrome...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152461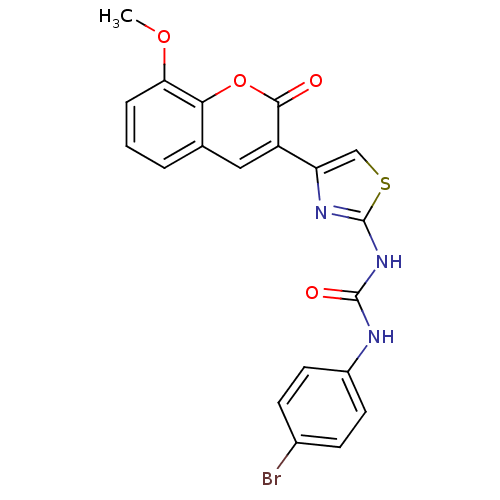 (1-(4-Bromophenyl)-3-(4-(8-methoxy-2-oxo-2H-chromen...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.91E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152460 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.06E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152441 (1-Phenyl-3-(4-(2-oxo-2H-chromen-3-yl)thiazol-2-yl)...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.32E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152441 (1-Phenyl-3-(4-(2-oxo-2H-chromen-3-yl)thiazol-2-yl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.47E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152442 (1-(4-Nitrophenyl)-3-(4-(2-oxo-2H-chromen-3-yl)thia...) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.48E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152438 (1-(3-Methoxyphenyl)-3-(4-(2-oxo-2H-chromen-3-yl)th...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152453 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.75E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152463 (1-(3-Methoxyphenyl)-3-(4-(6-nitro-2-oxo-2H-chromen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.75E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152468 (1-(4-Chlorophenyl)-3-(4-(6-nitro-2-oxo-2H-chromen-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.83E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152454 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.84E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152456 (1-(2-Fluorophenyl)-3-(4-(8-methoxy-2-oxo-2H-chrome...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.96E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152440 (1-(p-tolyl)-3-(4-(2-oxo-2H-chromen-3-yl)thiazol-2-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.15E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152452 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.21E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152439 (1-(4-Methoxyphenyl)-3-(4-(2-oxo-2H-chromen-3-yl)th...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.26E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152455 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.37E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152450 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.38E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152453 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.41E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152466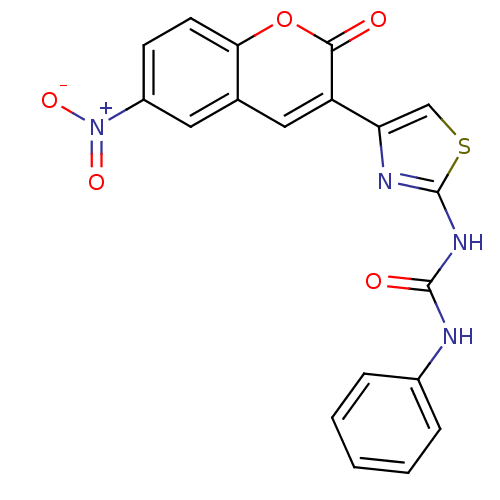 (1-(4-(6-Nitro-2-oxo-2H-chromen-3-yl)thiazol-2-yl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.45E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152445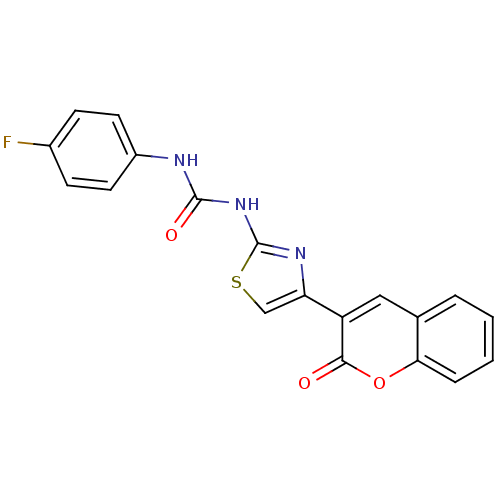 (1-(4-Fluorophenyl)-3-(4-(2-oxo-2H-chromen-3-yl)thi...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.49E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152447 (1-(4-Chlorophenyl)-3-(4-(2-oxo-2H-chromen-3-yl)thi...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.84E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152454 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152449 (1-(4-Iodophenyl)-3-(4-(2-oxo-2H-chromen-3-yl)thiaz...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.12E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152469 (1-(4-Bromophenyl)-3-(4-(6-nitro-2-oxo-2H-chromen-3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.25E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152465 (1-(4-(6-Nitro-2-oxo-2H-chromen-3-yl)thiazol-2-yl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.39E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152448 (1-(4-Bromophenyl)-3-(4-(2-oxo-2H-chromen-3-yl)thia...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.54E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152452 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.56E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152439 (1-(4-Methoxyphenyl)-3-(4-(2-oxo-2H-chromen-3-yl)th...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.59E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152470 (1-(4-Iodophenyl)-3-(4-(6-nitro-2-oxo-2H-chromen-3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.75E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152451 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.96E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152445 (1-(4-Fluorophenyl)-3-(4-(2-oxo-2H-chromen-3-yl)thi...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152466 (1-(4-(6-Nitro-2-oxo-2H-chromen-3-yl)thiazol-2-yl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152459 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152460 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152457 (1-(3-Fluorophenyl)-3-(4-(8-methoxy-2-oxo-2H-chrome...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152446 (1-(3-Chlorophenyl)-3-(4-(2-oxo-2H-chromen-3-yl)thi...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.11E+5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152449 (1-(4-Iodophenyl)-3-(4-(2-oxo-2H-chromen-3-yl)thiaz...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152458 (1-(4-Fluorophenyl)-3-(4-(8-methoxy-2-oxo-2H-chrome...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.15E+5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152461 (1-(4-Bromophenyl)-3-(4-(8-methoxy-2-oxo-2H-chromen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152464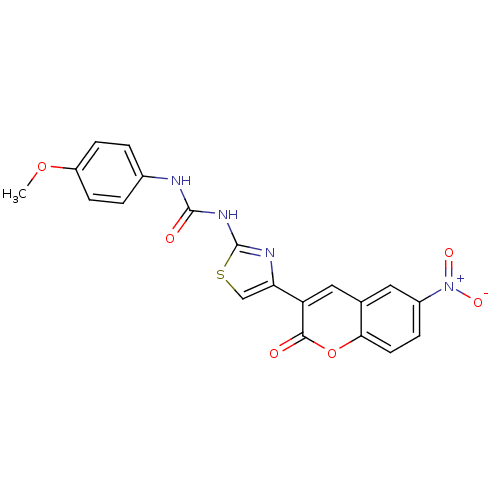 (1-(4-Methoxyphenyl)-3-(4-(6-nitro-2-oxo-2H-chromen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152451 (1-(4-(8-Methoxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152448 (1-(4-Bromophenyl)-3-(4-(2-oxo-2H-chromen-3-yl)thia...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.21E+5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152468 (1-(4-Chlorophenyl)-3-(4-(6-nitro-2-oxo-2H-chromen-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.29E+5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152467 (1-(4-Fluorophenyl)-3-(4-(6-nitro-2-oxo-2H-chromen-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.35E+5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152462 (1-(4-Iodophenyl)-3-(4-(8-methoxy-2-oxo-2H-chromen-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM152465 (1-(4-(6-Nitro-2-oxo-2H-chromen-3-yl)thiazol-2-yl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.95E+5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM152464 (1-(4-Methoxyphenyl)-3-(4-(6-nitro-2-oxo-2H-chromen...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Bezmialem Vakif University | Assay Description The substrates of the reaction were acetylthiocholine iodide and butyrylthiocholine iodide. 5,5'-dithio-bis(2-nitrobenzoic) acid (DTNB) was used ... | Bioorg Chem 59: 80-90 (2015) Article DOI: 10.1016/j.bioorg.2015.02.002 BindingDB Entry DOI: 10.7270/Q2668BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||