

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093085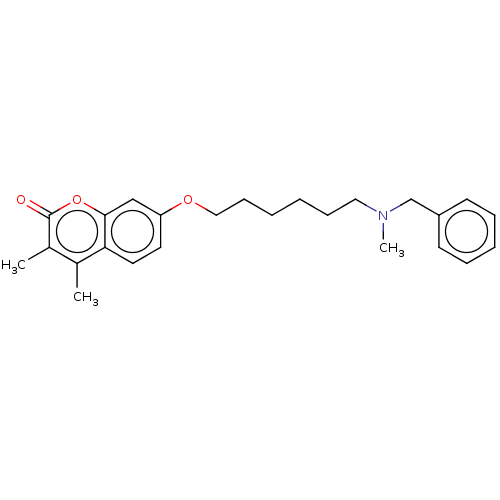 (CHEMBL3586582) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Mixed type inhibition of electric eel AChE by Lineweaver-Burk plot | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093227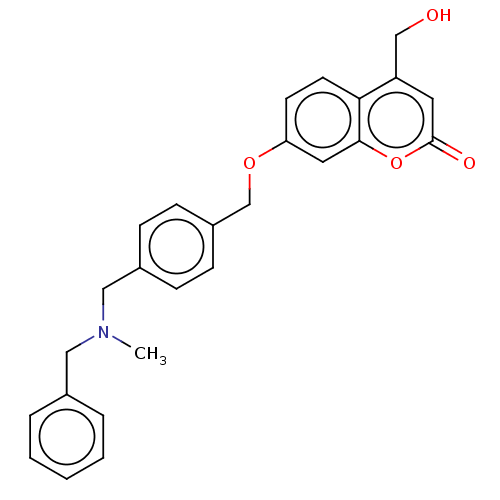 (CHEMBL3586608) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Mixed type inhibition of electric eel AChE by Lineweaver-Burk plot | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50093335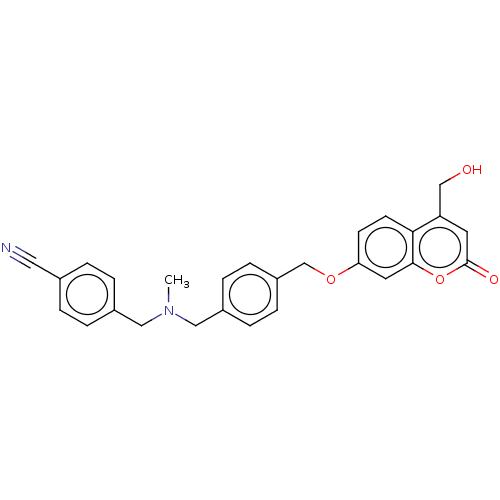 (CHEMBL3586611) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50093227 (CHEMBL3586608) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50093329 (CHEMBL3586603) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50093237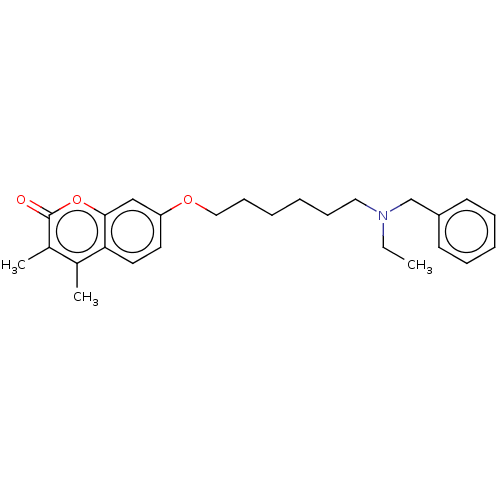 (CHEMBL3586583) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50093332 (CHEMBL3586605) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50093233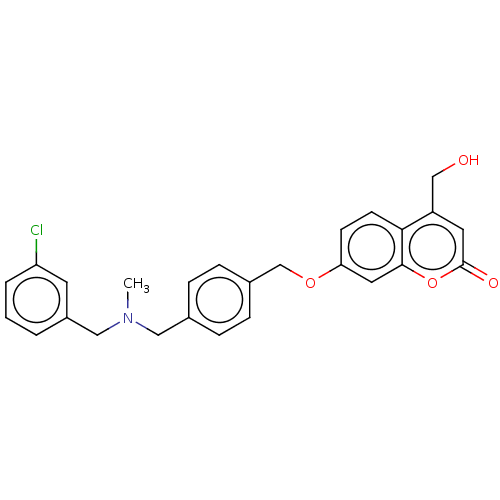 (CHEMBL3586610) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50093084 (CHEMBL3586577) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093335 (CHEMBL3586611) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50093330 (CHEMBL3586604) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50093212 (CHEMBL3586592) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50093230 (CHEMBL3586602) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50093238 (CHEMBL3586595) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093085 (CHEMBL3586582) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50093236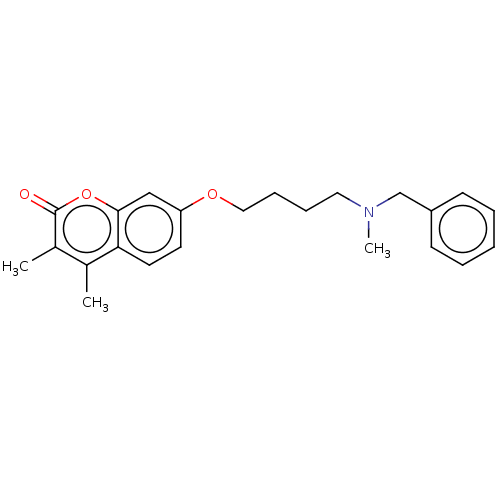 (CHEMBL3586576) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093238 (CHEMBL3586595) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50093237 (CHEMBL3586583) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093326 (CHEMBL3586600) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093227 (CHEMBL3586608) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50172756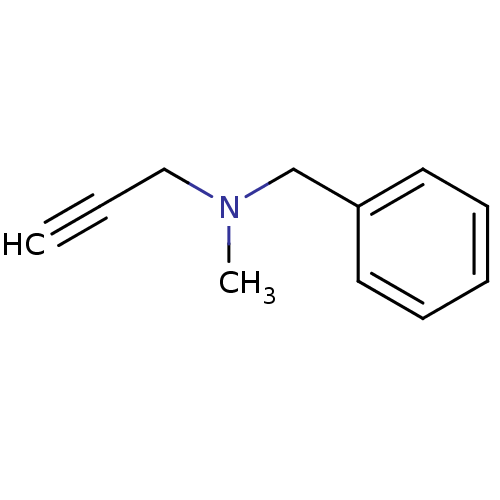 (Benzyl-methyl-prop-2-ynyl-amine | CHEMBL673 | Euto...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50093240 (CHEMBL3586574) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093322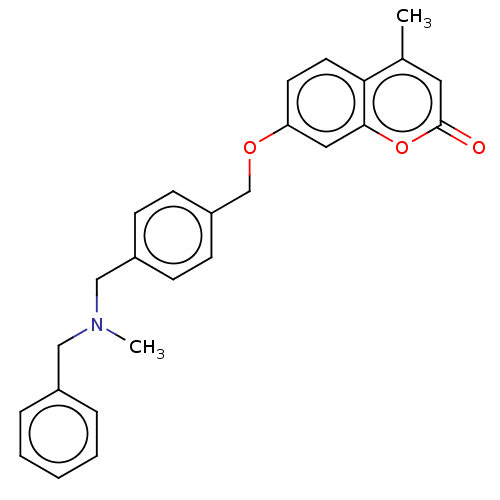 (CHEMBL3586594) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50093085 (CHEMBL3586582) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 191 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093231 (CHEMBL3586606) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093294 (CHEMBL3586590) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093233 (CHEMBL3586610) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093233 (CHEMBL3586610) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093295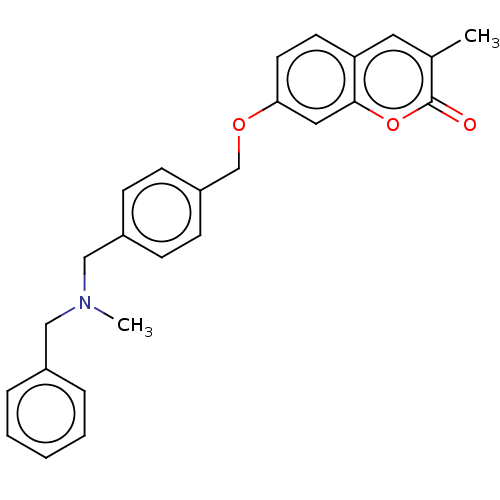 (CHEMBL3586593) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093230 (CHEMBL3586602) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093243 (CHEMBL3585361) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093084 (CHEMBL3586577) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093250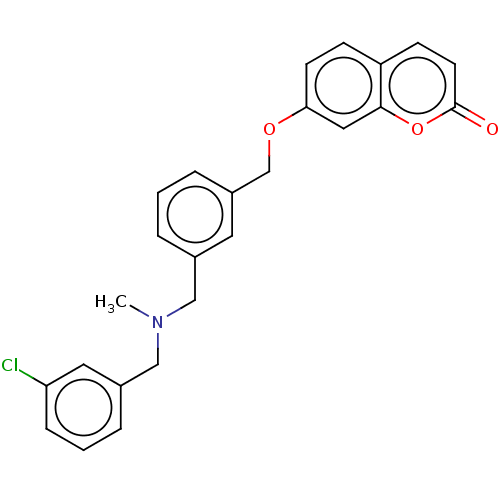 (CHEMBL3586589) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093237 (CHEMBL3586583) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50093085 (CHEMBL3586582) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 321 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093240 (CHEMBL3586574) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093233 (CHEMBL3586610) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093085 (CHEMBL3586582) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093239 (CHEMBL3586575) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50093084 (CHEMBL3586577) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093330 (CHEMBL3586604) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093227 (CHEMBL3586608) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093227 (CHEMBL3586608) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093232 (CHEMBL3586609) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093237 (CHEMBL3586583) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093238 (CHEMBL3586595) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50093236 (CHEMBL3586576) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093237 (CHEMBL3586583) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093083 (CHEMBL3586614) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093236 (CHEMBL3586576) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093084 (CHEMBL3586577) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093295 (CHEMBL3586593) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50093085 (CHEMBL3586582) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-A using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093234 (CHEMBL3586612) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093232 (CHEMBL3586609) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093241 (CHEMBL3586578) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093232 (CHEMBL3586609) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093229 (CHEMBL3586598) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093233 (CHEMBL3586610) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093235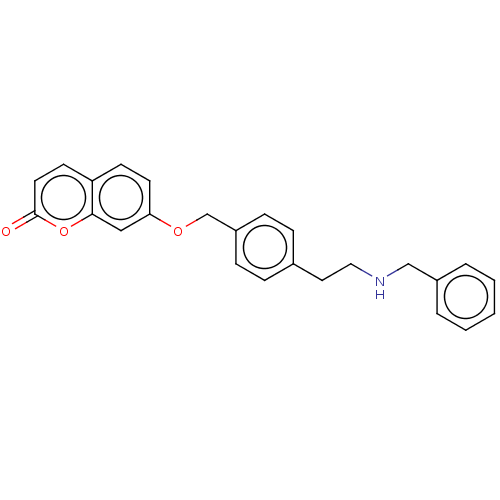 (CHEMBL3586613) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093327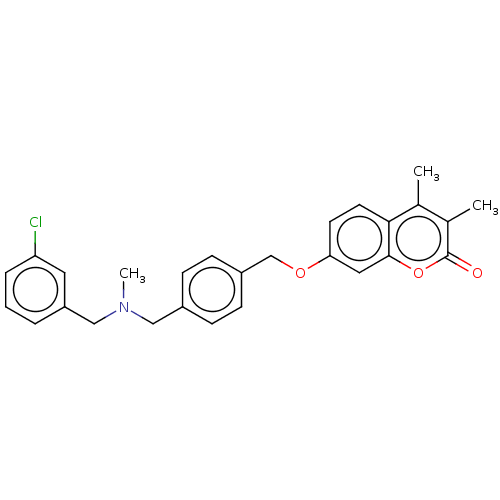 (CHEMBL3586601) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093085 (CHEMBL3586582) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093238 (CHEMBL3586595) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093237 (CHEMBL3586583) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093245 (CHEMBL3586581) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093212 (CHEMBL3586592) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093236 (CHEMBL3586576) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093212 (CHEMBL3586592) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093234 (CHEMBL3586612) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093249 (CHEMBL3586588) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50093212 (CHEMBL3586592) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093322 (CHEMBL3586594) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093084 (CHEMBL3586577) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093245 (CHEMBL3586581) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093212 (CHEMBL3586592) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50093085 (CHEMBL3586582) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50093084 (CHEMBL3586577) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093241 (CHEMBL3586578) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093239 (CHEMBL3586575) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093334 (CHEMBL3586607) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093247 (CHEMBL3586586) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093326 (CHEMBL3586600) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093322 (CHEMBL3586594) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093240 (CHEMBL3586574) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093295 (CHEMBL3586593) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093227 (CHEMBL3586608) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093236 (CHEMBL3586576) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093238 (CHEMBL3586595) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093229 (CHEMBL3586598) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093324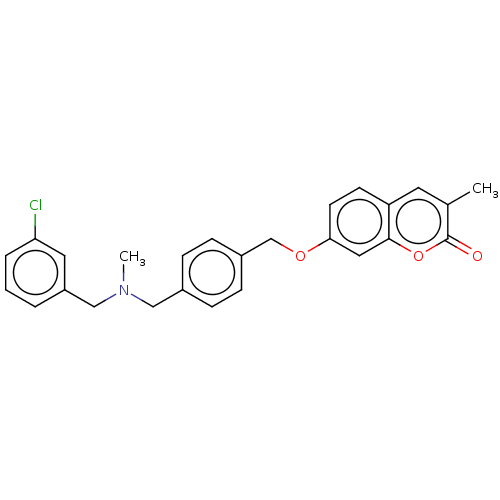 (CHEMBL3586599) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093240 (CHEMBL3586574) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093228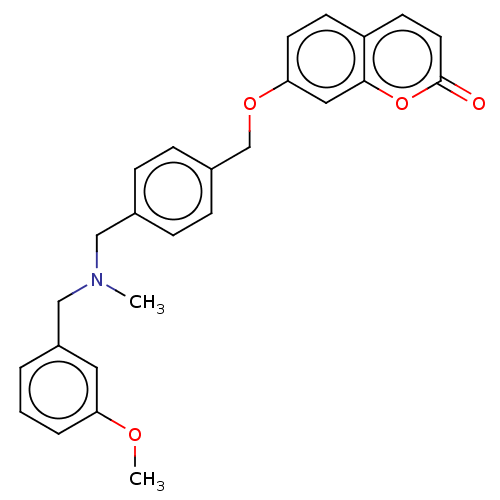 (CHEMBL3586597) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093083 (CHEMBL3586614) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093085 (CHEMBL3586582) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50093235 (CHEMBL3586613) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-A using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093338 (CHEMBL3586585) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093329 (CHEMBL3586603) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093083 (CHEMBL3586614) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093084 (CHEMBL3586577) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093247 (CHEMBL3586586) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50093237 (CHEMBL3586583) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-A using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50093084 (CHEMBL3586577) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-A using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093247 (CHEMBL3586586) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093242 (CHEMBL3586579) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093295 (CHEMBL3586593) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093249 (CHEMBL3586588) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093242 (CHEMBL3586579) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093243 (CHEMBL3585361) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093229 (CHEMBL3586598) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093244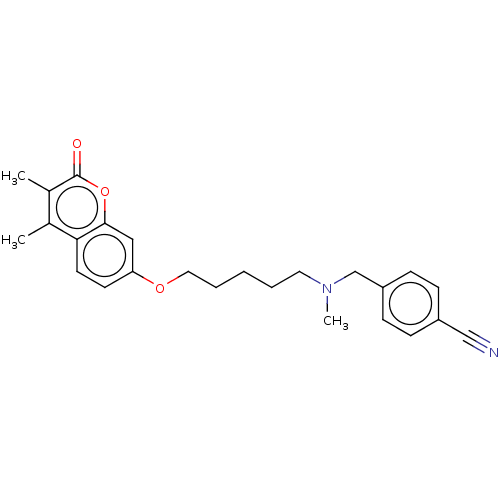 (CHEMBL3586580) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093248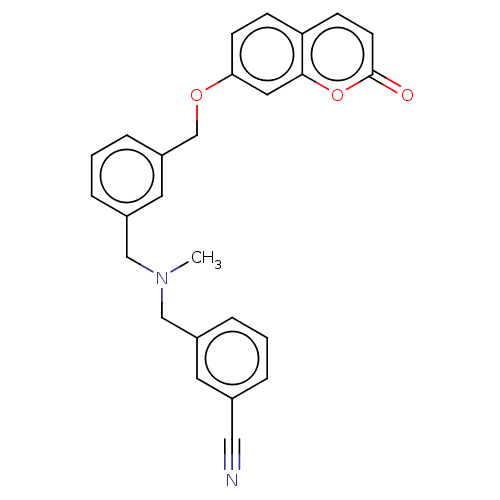 (CHEMBL3586587) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093323 (CHEMBL3586596) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093241 (CHEMBL3586578) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093339 (CHEMBL3586591) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093228 (CHEMBL3586597) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093332 (CHEMBL3586605) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093246 (CHEMBL3586584) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093244 (CHEMBL3586580) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093330 (CHEMBL3586604) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093327 (CHEMBL3586601) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093250 (CHEMBL3586589) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093339 (CHEMBL3586591) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093294 (CHEMBL3586590) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093236 (CHEMBL3586576) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093248 (CHEMBL3586587) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093332 (CHEMBL3586605) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093239 (CHEMBL3586575) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50172756 (Benzyl-methyl-prop-2-ynyl-amine | CHEMBL673 | Euto...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093322 (CHEMBL3586594) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093323 (CHEMBL3586596) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093242 (CHEMBL3586579) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093234 (CHEMBL3586612) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50093241 (CHEMBL3586578) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-A using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093248 (CHEMBL3586587) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093332 (CHEMBL3586605) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093244 (CHEMBL3586580) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50093238 (CHEMBL3586595) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093230 (CHEMBL3586602) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50093335 (CHEMBL3586611) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50093083 (CHEMBL3586614) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093324 (CHEMBL3586599) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093228 (CHEMBL3586597) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093235 (CHEMBL3586613) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093326 (CHEMBL3586600) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093246 (CHEMBL3586584) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50093242 (CHEMBL3586579) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-A using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093231 (CHEMBL3586606) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093230 (CHEMBL3586602) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093250 (CHEMBL3586589) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093230 (CHEMBL3586602) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093335 (CHEMBL3586611) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093249 (CHEMBL3586588) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50093236 (CHEMBL3586576) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-A using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093338 (CHEMBL3586585) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093329 (CHEMBL3586603) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093294 (CHEMBL3586590) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093323 (CHEMBL3586596) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50093239 (CHEMBL3586575) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-A using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093240 (CHEMBL3586574) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093245 (CHEMBL3586581) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093330 (CHEMBL3586604) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50093243 (CHEMBL3585361) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50093227 (CHEMBL3586608) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50093245 (CHEMBL3586581) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-A using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50093246 (CHEMBL3586584) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-A using kynuramine substrate in rat mitochondrial substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50093212 (CHEMBL3586592) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by spectrophotometric Ellman's method | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50093233 (CHEMBL3586610) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50093227 (CHEMBL3586608) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||