

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50069812 (CHEMBL3407784) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adenosine A3 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50116875 (CHEMBL3612943) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adenosine A3 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50116885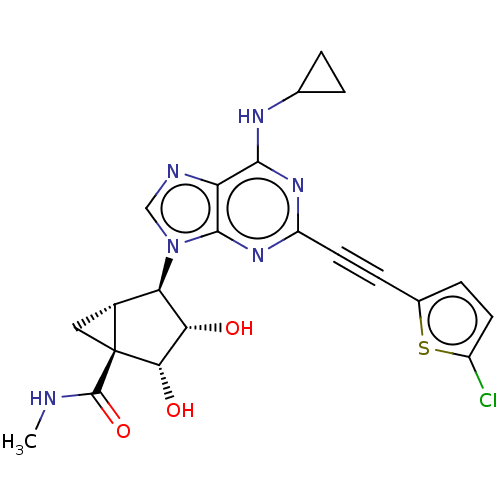 (CHEMBL3612935) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adenosine A3 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50116882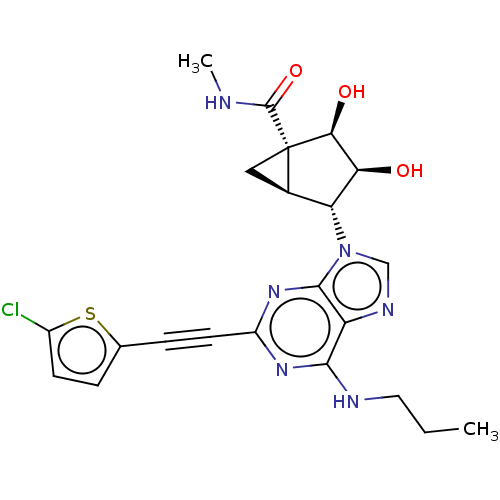 (CHEMBL3612932) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adenosine A3 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50116871 (CHEMBL3612944) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adenosine A3 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Canis lupus familiaris) | BDBM50116883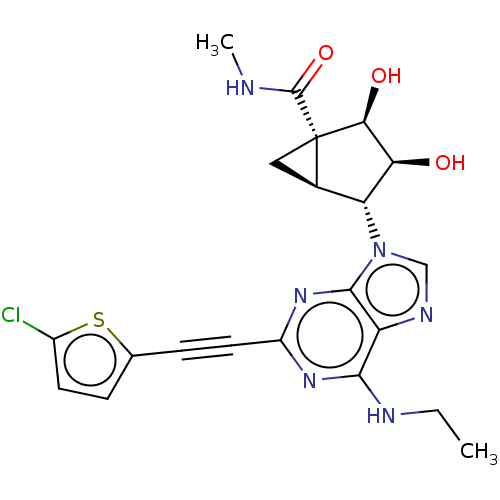 (CHEMBL3612931) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to canine adenosine A3 receptor expressed in HEK293 cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50116878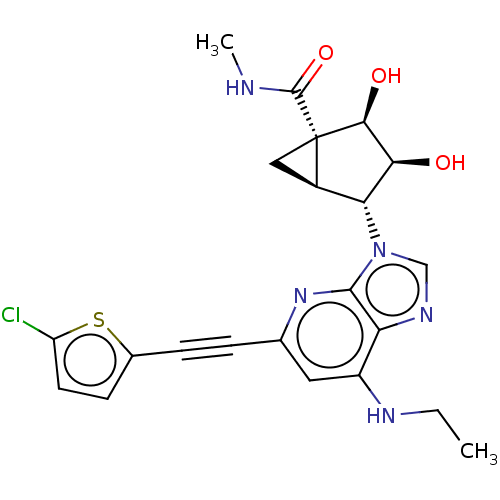 (CHEMBL3612940) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adenosine A3 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50116887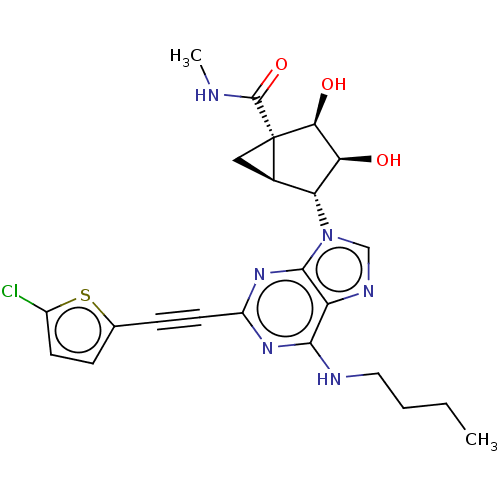 (CHEMBL3612933) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adenosine A3 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50116886 (CHEMBL3612934) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adenosine A3 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50116879 (CHEMBL3612939) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adenosine A3 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50116883 (CHEMBL3612931) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adenosine A3 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50116884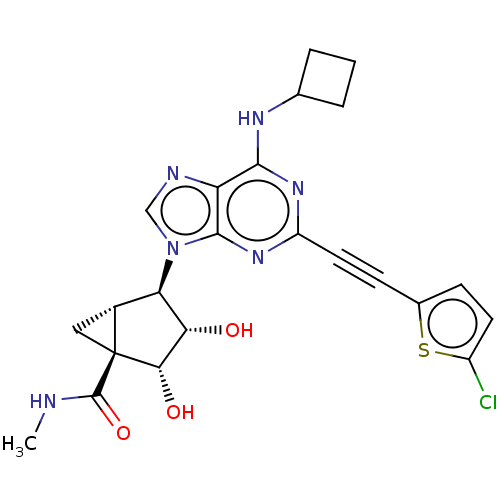 (CHEMBL3612936) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adenosine A3 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Canis lupus familiaris) | BDBM50116882 (CHEMBL3612932) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to canine adenosine A3 receptor expressed in HEK293 cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Mus musculus) | BDBM50116882 (CHEMBL3612932) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to mouse adenosine A3 receptor expressed in HEK293 cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50116881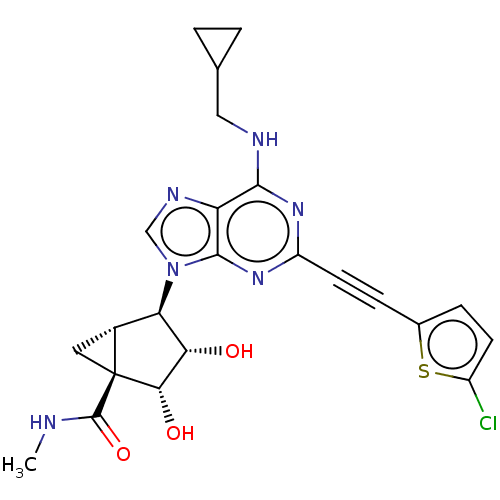 (CHEMBL3612937) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adenosine A3 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50116863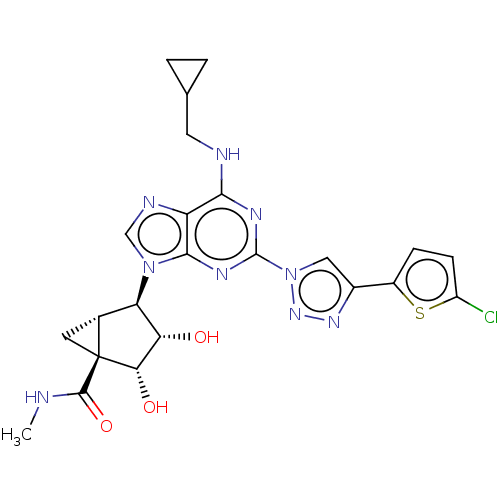 (CHEMBL3612945) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adenosine A3 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50116876 (CHEMBL3612942) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adenosine A3 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50116880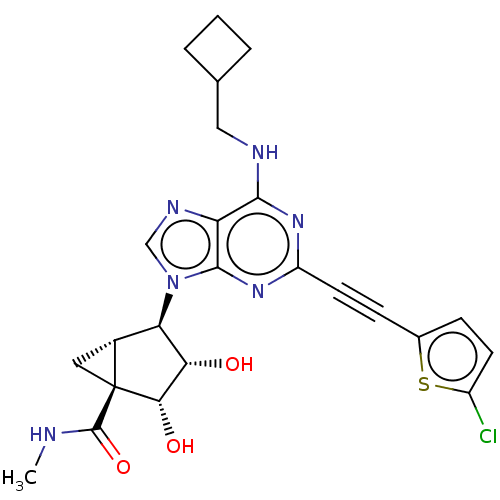 (CHEMBL3612938) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adenosine A3 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Mus musculus) | BDBM50116878 (CHEMBL3612940) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to mouse adenosine A3 receptor expressed in HEK293 cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50116854 (CHEMBL3612946) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adenosine A3 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Mus musculus) | BDBM50116883 (CHEMBL3612931) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to mouse adenosine A3 receptor expressed in HEK293 cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Mus musculus) | BDBM50116879 (CHEMBL3612939) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to mouse adenosine A3 receptor expressed in HEK293 cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50116877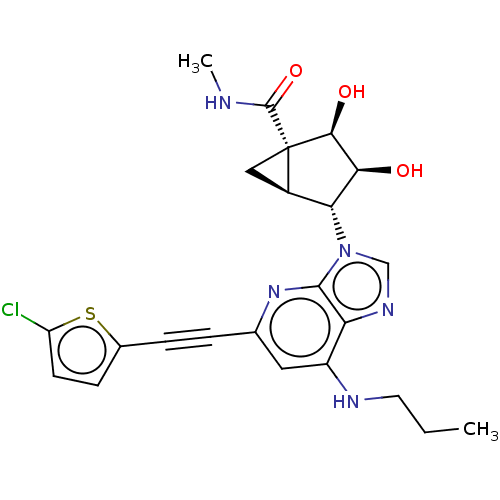 (CHEMBL3612941) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adenosine A3 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Canis lupus familiaris) | BDBM50116878 (CHEMBL3612940) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to canine adenosine A3 receptor expressed in HEK293 cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Canis lupus familiaris) | BDBM50116879 (CHEMBL3612939) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to canine adenosine A3 receptor expressed in HEK293 cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50116853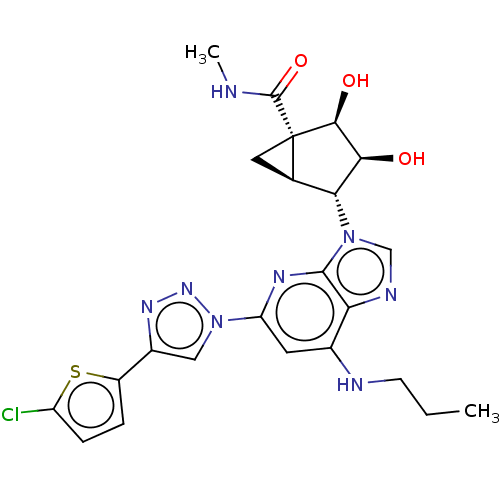 (CHEMBL3612947) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adenosine A3 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50116854 (CHEMBL3612946) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]N6-R-phenylisopropyladenosine from human adenosine A1 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50116863 (CHEMBL3612945) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]N6-R-phenylisopropyladenosine from human adenosine A1 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50116877 (CHEMBL3612941) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]N6-R-phenylisopropyladenosine from human adenosine A1 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50116878 (CHEMBL3612940) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to 5HT2B receptor (unknown origin) | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Canis lupus familiaris) | BDBM50069812 (CHEMBL3407784) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to canine adenosine A3 receptor expressed in HEK293 cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Mus musculus) | BDBM50069812 (CHEMBL3407784) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to mouse adenosine A3 receptor expressed in HEK293 cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50407975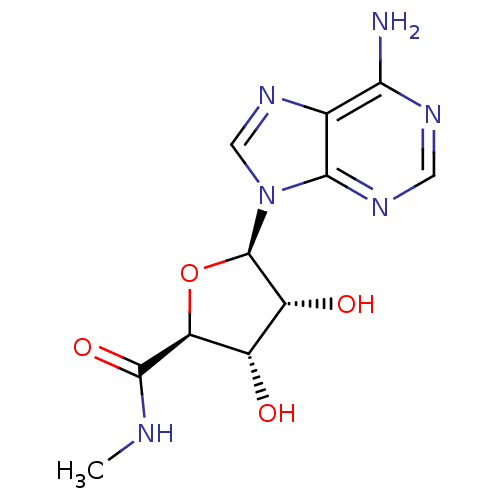 (CHEMBL519809) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 38 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A3 receptor expressed in CHO cells assessed as inhibition of cAMP production | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50116878 (CHEMBL3612940) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A3 receptor expressed in CHO cells assessed as inhibition of cAMP production | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||