 Found 24 hits of Enzyme Inhibition Constant Data
Found 24 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50264106

(CHEMBL3818875)Show SMILES CNc1nc(C)nc(N[C@H]2CCC[C@H](C2)C(=O)NCc2ccc(cc2C(F)(F)F)C#N)n1 |r| Show InChI InChI=1S/C21H24F3N7O/c1-12-28-19(26-2)31-20(29-12)30-16-5-3-4-14(9-16)18(32)27-11-15-7-6-13(10-25)8-17(15)21(22,23)24/h6-8,14,16H,3-5,9,11H2,1-2H3,(H,27,32)(H2,26,28,29,30,31)/t14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sEH using 14,15-epoxy-5Z,8Z,11Z-eicosatrienoic acid as substrate assessed as formation of 14,15-dihydroxy-5Z,8Z,11Zei... |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50264106

(CHEMBL3818875)Show SMILES CNc1nc(C)nc(N[C@H]2CCC[C@H](C2)C(=O)NCc2ccc(cc2C(F)(F)F)C#N)n1 |r| Show InChI InChI=1S/C21H24F3N7O/c1-12-28-19(26-2)31-20(29-12)30-16-5-3-4-14(9-16)18(32)27-11-15-7-6-13(10-25)8-17(15)21(22,23)24/h6-8,14,16H,3-5,9,11H2,1-2H3,(H,27,32)(H2,26,28,29,30,31)/t14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sEH using 14,15-epoxy-5Z,8Z,11Z-eicosatrienoic acid as substrate assessed as formation of 14,15-dihydroxy-5Z,8Z,11Zei... |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50519649

(CHEMBL4439913)Show SMILES CNC(=O)c1ccc2n(cnc2c1)-c1ccc(CC(=O)OC(C)(C)C)cc1 Show InChI InChI=1S/C21H23N3O3/c1-21(2,3)27-19(25)11-14-5-8-16(9-6-14)24-13-23-17-12-15(20(26)22-4)7-10-18(17)24/h5-10,12-13H,11H2,1-4H3,(H,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant RIP3 (2 to 328 residues) expressed in baculovirus by ADP-glo assay |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50519649

(CHEMBL4439913)Show SMILES CNC(=O)c1ccc2n(cnc2c1)-c1ccc(CC(=O)OC(C)(C)C)cc1 Show InChI InChI=1S/C21H23N3O3/c1-21(2,3)27-19(25)11-14-5-8-16(9-6-14)24-13-23-17-12-15(20(26)22-4)7-10-18(17)24/h5-10,12-13H,11H2,1-4H3,(H,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant RIP3 (2 to 328 residues) expressed in baculovirus by ADP-glo assay |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
2',5'-phosphodiesterase 12
(Homo sapiens) | BDBM50532283
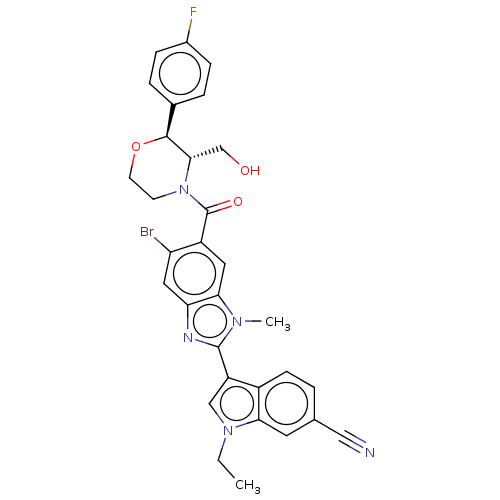
(CHEMBL4556129)Show SMILES CCn1cc(-c2nc3cc(Br)c(cc3n2C)C(=O)N2CCO[C@H]([C@@H]2CO)c2ccc(F)cc2)c2ccc(cc12)C#N |r| Show InChI InChI=1S/C31H27BrFN5O3/c1-3-37-16-23(21-9-4-18(15-34)12-26(21)37)30-35-25-14-24(32)22(13-27(25)36(30)2)31(40)38-10-11-41-29(28(38)17-39)19-5-7-20(33)8-6-19/h4-9,12-14,16,28-29,39H,3,10-11,17H2,1-2H3/t28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of human PDE12 (17 to 609 residues) expresssed in Escherichia coli BL21(DE3) cells using 2-5A as substrate assessed as AMP monomers and AT... |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
2',5'-phosphodiesterase 12
(Homo sapiens) | BDBM50532283

(CHEMBL4556129)Show SMILES CCn1cc(-c2nc3cc(Br)c(cc3n2C)C(=O)N2CCO[C@H]([C@@H]2CO)c2ccc(F)cc2)c2ccc(cc12)C#N |r| Show InChI InChI=1S/C31H27BrFN5O3/c1-3-37-16-23(21-9-4-18(15-34)12-26(21)37)30-35-25-14-24(32)22(13-27(25)36(30)2)31(40)38-10-11-41-29(28(38)17-39)19-5-7-20(33)8-6-19/h4-9,12-14,16,28-29,39H,3,10-11,17H2,1-2H3/t28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of human PDE12 (17 to 609 residues) expresssed in Escherichia coli BL21(DE3) cells using 2-5A as substrate assessed as AMP monomers and AT... |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50532278
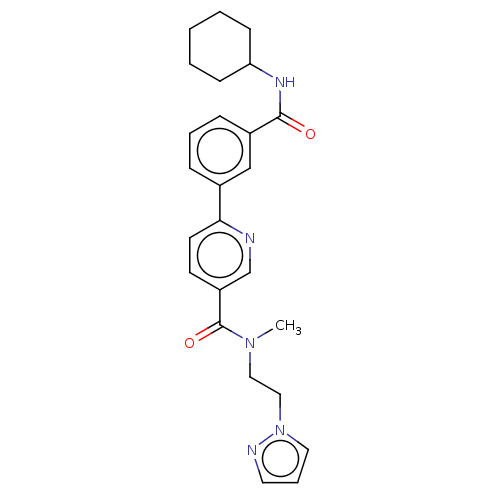
(CHEMBL4452411)Show SMILES CN(CCn1cccn1)C(=O)c1ccc(nc1)-c1cccc(c1)C(=O)NC1CCCCC1 Show InChI InChI=1S/C25H29N5O2/c1-29(15-16-30-14-6-13-27-30)25(32)21-11-12-23(26-18-21)19-7-5-8-20(17-19)24(31)28-22-9-3-2-4-10-22/h5-8,11-14,17-18,22H,2-4,9-10,15-16H2,1H3,(H,28,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His-tagged human recombinant sEH expressed in insect Sf21 cells using Epoxy Fluor 7 as substrate preincubated fo... |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50532278

(CHEMBL4452411)Show SMILES CN(CCn1cccn1)C(=O)c1ccc(nc1)-c1cccc(c1)C(=O)NC1CCCCC1 Show InChI InChI=1S/C25H29N5O2/c1-29(15-16-30-14-6-13-27-30)25(32)21-11-12-23(26-18-21)19-7-5-8-20(17-19)24(31)28-22-9-3-2-4-10-22/h5-8,11-14,17-18,22H,2-4,9-10,15-16H2,1H3,(H,28,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His-tagged human recombinant sEH expressed in insect Sf21 cells using Epoxy Fluor 7 as substrate preincubated fo... |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Gallus gallus (Chicken)) | BDBM50532280

(CHEMBL4465847)Show SMILES [H][C@]1(CCNC(=O)[C@H](CCCNC(=O)c2cnccn2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC2CCCCC2)NC(=O)\C=C/C(=O)N1)C(O)=O |r,c:48| Show InChI InChI=1S/C36H46N8O8/c45-30-13-14-31(46)42-27(20-23-8-3-1-4-9-23)34(49)44-28(21-24-10-5-2-6-11-24)35(50)43-25(32(47)40-17-15-26(41-30)36(51)52)12-7-16-39-33(48)29-22-37-18-19-38-29/h2,5-6,10-11,13-14,18-19,22-23,25-28H,1,3-4,7-9,12,15-17,20-21H2,(H,39,48)(H,40,47)(H,41,45)(H,42,46)(H,43,50)(H,44,49)(H,51,52)/b14-13-/t25-,26-,27-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of chicken Src kinase domain using AEEEIYGEFAKKK as substrate by continuous spectrophotometric assay |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Gallus gallus (Chicken)) | BDBM50532280

(CHEMBL4465847)Show SMILES [H][C@]1(CCNC(=O)[C@H](CCCNC(=O)c2cnccn2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC2CCCCC2)NC(=O)\C=C/C(=O)N1)C(O)=O |r,c:48| Show InChI InChI=1S/C36H46N8O8/c45-30-13-14-31(46)42-27(20-23-8-3-1-4-9-23)34(49)44-28(21-24-10-5-2-6-11-24)35(50)43-25(32(47)40-17-15-26(41-30)36(51)52)12-7-16-39-33(48)29-22-37-18-19-38-29/h2,5-6,10-11,13-14,18-19,22-23,25-28H,1,3-4,7-9,12,15-17,20-21H2,(H,39,48)(H,40,47)(H,41,45)(H,42,46)(H,43,50)(H,44,49)(H,51,52)/b14-13-/t25-,26-,27-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of chicken Src kinase domain using AEEEIYGEFAKKK as substrate by continuous spectrophotometric assay |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1D
(Homo sapiens (Human)) | BDBM50118478
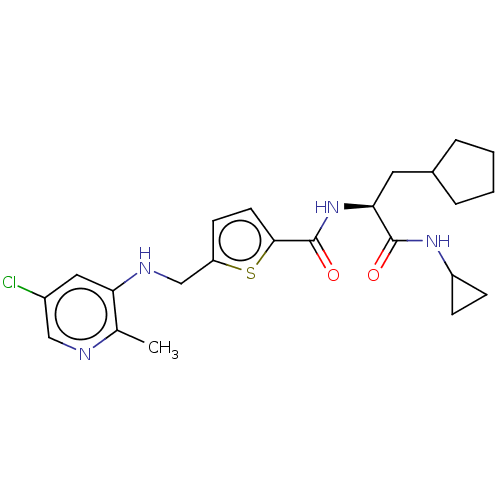
(CHEMBL3613749)Show SMILES Cc1ncc(Cl)cc1NCc1ccc(s1)C(=O)N[C@@H](CC1CCCC1)C(=O)NC1CC1 |r| Show InChI InChI=1S/C23H29ClN4O2S/c1-14-19(11-16(24)12-25-14)26-13-18-8-9-21(31-18)23(30)28-20(10-15-4-2-3-5-15)22(29)27-17-6-7-17/h8-9,11-12,15,17,20,26H,2-7,10,13H2,1H3,(H,27,29)(H,28,30)/t20-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of human Wip1 (2 to 420 residues) expressed in baculovirus-infected insect SF9 cells assessed as fluorescein diphosphate hydrolysis after ... |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1D
(Homo sapiens (Human)) | BDBM50118478

(CHEMBL3613749)Show SMILES Cc1ncc(Cl)cc1NCc1ccc(s1)C(=O)N[C@@H](CC1CCCC1)C(=O)NC1CC1 |r| Show InChI InChI=1S/C23H29ClN4O2S/c1-14-19(11-16(24)12-25-14)26-13-18-8-9-21(31-18)23(30)28-20(10-15-4-2-3-5-15)22(29)27-17-6-7-17/h8-9,11-12,15,17,20,26H,2-7,10,13H2,1H3,(H,27,29)(H,28,30)/t20-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of human Wip1 (2 to 420 residues) expressed in baculovirus-infected insect SF9 cells assessed as fluorescein diphosphate hydrolysis after ... |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50532279
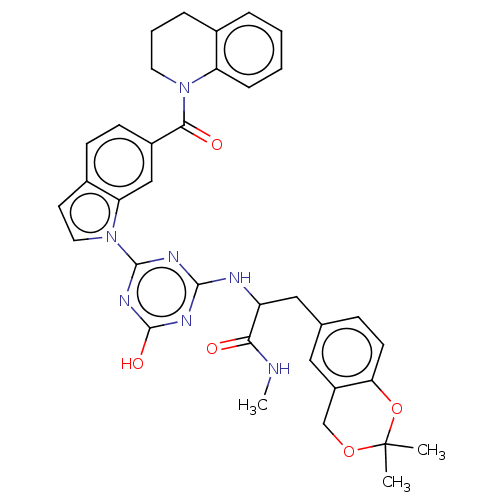
(CHEMBL4463696)Show SMILES CNC(=O)C(Cc1ccc2OC(C)(C)OCc2c1)Nc1nc(O)nc(n1)-n1ccc2ccc(cc12)C(=O)N1CCCc2ccccc12 Show InChI InChI=1S/C35H35N7O5/c1-35(2)46-20-25-17-21(10-13-29(25)47-35)18-26(30(43)36-3)37-32-38-33(40-34(45)39-32)42-16-14-23-11-12-24(19-28(23)42)31(44)41-15-6-8-22-7-4-5-9-27(22)41/h4-5,7,9-14,16-17,19,26H,6,8,15,18,20H2,1-3H3,(H,36,43)(H2,37,38,39,40,45) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of p38 MAPK (unknown origin) |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50532279

(CHEMBL4463696)Show SMILES CNC(=O)C(Cc1ccc2OC(C)(C)OCc2c1)Nc1nc(O)nc(n1)-n1ccc2ccc(cc12)C(=O)N1CCCc2ccccc12 Show InChI InChI=1S/C35H35N7O5/c1-35(2)46-20-25-17-21(10-13-29(25)47-35)18-26(30(43)36-3)37-32-38-33(40-34(45)39-32)42-16-14-23-11-12-24(19-28(23)42)31(44)41-15-6-8-22-7-4-5-9-27(22)41/h4-5,7,9-14,16-17,19,26H,6,8,15,18,20H2,1-3H3,(H,36,43)(H2,37,38,39,40,45) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of p38 MAPK (unknown origin) |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Protein-arginine deiminase type-4
(Homo sapiens (Human)) | BDBM50532282
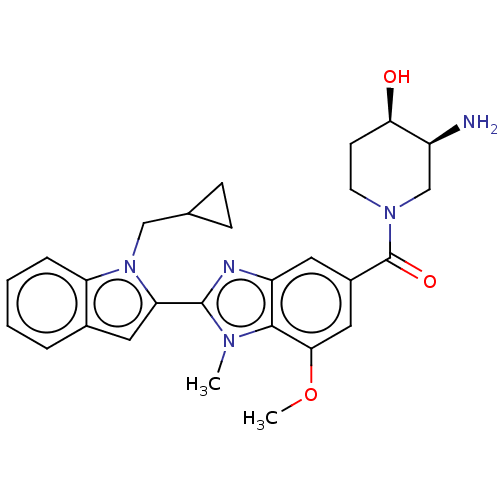
(CHEMBL4539512)Show SMILES COc1cc(cc2nc(-c3cc4ccccc4n3CC3CC3)n(C)c12)C(=O)N1CC[C@@H](O)[C@@H](N)C1 |r| Show InChI InChI=1S/C27H31N5O3/c1-30-25-20(11-18(13-24(25)35-2)27(34)31-10-9-23(33)19(28)15-31)29-26(30)22-12-17-5-3-4-6-21(17)32(22)14-16-7-8-16/h3-6,11-13,16,19,23,33H,7-10,14-15,28H2,1-2H3/t19-,23+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Displacement of GSK215 from full length PAD4 (unknown origin) after 50 mins by fluorescence polarization assay in absence of calcium |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50525489

(CHEMBL4556893)Show SMILES NCCCC[C@@H]1NC(=O)\C=C\C(=O)N[C@@H](CCCCNC(=O)[C@@H](Cc2ccc(cc2)C(=O)c2ccccc2)NC(=O)[C@H](CC2CCCCC2)NC1=O)C(N)=O |r,t:9| Show InChI InChI=1S/C41H55N7O7/c42-23-9-7-16-32-40(54)48-34(25-27-11-3-1-4-12-27)41(55)47-33(26-28-17-19-30(20-18-28)37(51)29-13-5-2-6-14-29)39(53)44-24-10-8-15-31(38(43)52)45-35(49)21-22-36(50)46-32/h2,5-6,13-14,17-22,27,31-34H,1,3-4,7-12,15-16,23-26,42H2,(H2,43,52)(H,44,53)(H,45,49)(H,46,50)(H,47,55)(H,48,54)/b22-21+/t31-,32-,33+,34-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of IDE (unknown origin) assessed as cleavage of Mca-RPPGFSAFK(Dnp)-OH by fluorescence assay |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Protein-arginine deiminase type-4
(Homo sapiens (Human)) | BDBM50532282

(CHEMBL4539512)Show SMILES COc1cc(cc2nc(-c3cc4ccccc4n3CC3CC3)n(C)c12)C(=O)N1CC[C@@H](O)[C@@H](N)C1 |r| Show InChI InChI=1S/C27H31N5O3/c1-30-25-20(11-18(13-24(25)35-2)27(34)31-10-9-23(33)19(28)15-31)29-26(30)22-12-17-5-3-4-6-21(17)32(22)14-16-7-8-16/h3-6,11-13,16,19,23,33H,7-10,14-15,28H2,1-2H3/t19-,23+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Displacement of GSK215 from full length PAD4 (unknown origin) after 50 mins by fluorescence polarization assay in absence of calcium |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50525489

(CHEMBL4556893)Show SMILES NCCCC[C@@H]1NC(=O)\C=C\C(=O)N[C@@H](CCCCNC(=O)[C@@H](Cc2ccc(cc2)C(=O)c2ccccc2)NC(=O)[C@H](CC2CCCCC2)NC1=O)C(N)=O |r,t:9| Show InChI InChI=1S/C41H55N7O7/c42-23-9-7-16-32-40(54)48-34(25-27-11-3-1-4-12-27)41(55)47-33(26-28-17-19-30(20-18-28)37(51)29-13-5-2-6-14-29)39(53)44-24-10-8-15-31(38(43)52)45-35(49)21-22-36(50)46-32/h2,5-6,13-14,17-22,27,31-34H,1,3-4,7-12,15-16,23-26,42H2,(H2,43,52)(H,44,53)(H,45,49)(H,46,50)(H,47,55)(H,48,54)/b22-21+/t31-,32-,33+,34-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of IDE (unknown origin) assessed as cleavage of Mca-RPPGFSAFK(Dnp)-OH by fluorescence assay |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM36470
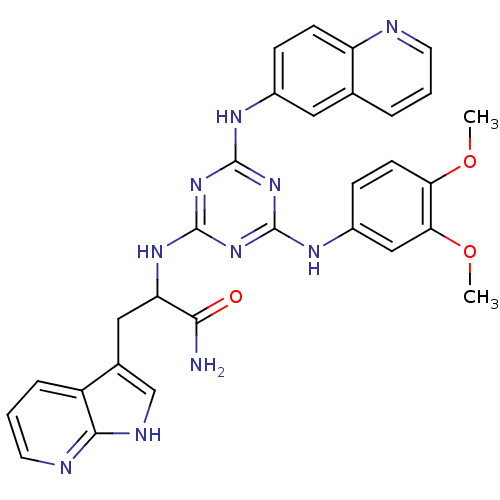
(2-(4-(3,4-Dimethoxyphenylamino)-6-(quinolin-6-ylam...)Show SMILES COc1ccc(Nc2nc(NC(Cc3c[nH]c4ncccc34)C(N)=O)nc(Nc3ccc4ncccc4c3)n2)cc1OC Show InChI InChI=1S/C30H28N10O3/c1-42-24-10-8-20(15-25(24)43-2)36-29-38-28(35-19-7-9-22-17(13-19)5-3-11-32-22)39-30(40-29)37-23(26(31)41)14-18-16-34-27-21(18)6-4-12-33-27/h3-13,15-16,23H,14H2,1-2H3,(H2,31,41)(H,33,34)(H3,35,36,37,38,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of aurora A kinase (unknown origin) |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM36470

(2-(4-(3,4-Dimethoxyphenylamino)-6-(quinolin-6-ylam...)Show SMILES COc1ccc(Nc2nc(NC(Cc3c[nH]c4ncccc34)C(N)=O)nc(Nc3ccc4ncccc4c3)n2)cc1OC Show InChI InChI=1S/C30H28N10O3/c1-42-24-10-8-20(15-25(24)43-2)36-29-38-28(35-19-7-9-22-17(13-19)5-3-11-32-22)39-30(40-29)37-23(26(31)41)14-18-16-34-27-21(18)6-4-12-33-27/h3-13,15-16,23H,14H2,1-2H3,(H2,31,41)(H,33,34)(H3,35,36,37,38,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of aurora A kinase (unknown origin) |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50379281
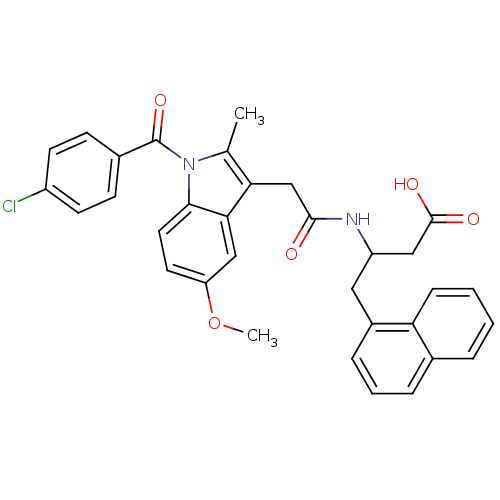
(CHEMBL2011504)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)NC(CC(O)=O)Cc3cccc4ccccc34)c2c1 Show InChI InChI=1S/C33H29ClN2O5/c1-20-28(29-18-26(41-2)14-15-30(29)36(20)33(40)22-10-12-24(34)13-11-22)19-31(37)35-25(17-32(38)39)16-23-8-5-7-21-6-3-4-9-27(21)23/h3-15,18,25H,16-17,19H2,1-2H3,(H,35,37)(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Binding affinity to human Bcl-xL after 1 hr by fluorescence polarization assay |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Interleukin-2
(Homo sapiens (Human)) | BDBM50532281
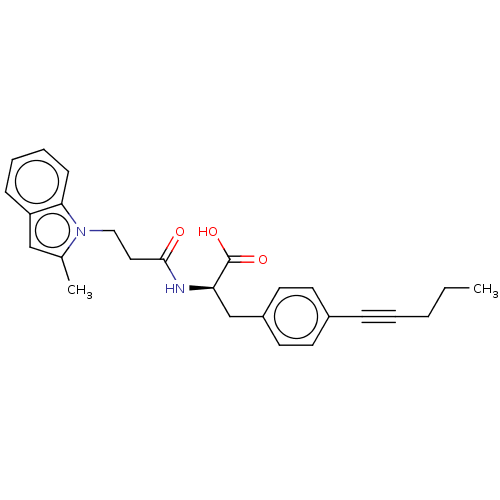
(CHEMBL4515387)Show SMILES CCCC#Cc1ccc(C[C@@H](NC(=O)CCn2c(C)cc3ccccc23)C(O)=O)cc1 |r| Show InChI InChI=1S/C26H28N2O3/c1-3-4-5-8-20-11-13-21(14-12-20)18-23(26(30)31)27-25(29)15-16-28-19(2)17-22-9-6-7-10-24(22)28/h6-7,9-14,17,23H,3-4,15-16,18H2,1-2H3,(H,27,29)(H,30,31)/t23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Binding affinity to human IL-2 measured over 30 mins by fluorescence polarization assay |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50379281

(CHEMBL2011504)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)NC(CC(O)=O)Cc3cccc4ccccc34)c2c1 Show InChI InChI=1S/C33H29ClN2O5/c1-20-28(29-18-26(41-2)14-15-30(29)36(20)33(40)22-10-12-24(34)13-11-22)19-31(37)35-25(17-32(38)39)16-23-8-5-7-21-6-3-4-9-27(21)23/h3-15,18,25H,16-17,19H2,1-2H3,(H,35,37)(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Binding affinity to human Bcl-xL after 1 hr by fluorescence polarization assay |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Interleukin-2
(Homo sapiens (Human)) | BDBM50532281

(CHEMBL4515387)Show SMILES CCCC#Cc1ccc(C[C@@H](NC(=O)CCn2c(C)cc3ccccc23)C(O)=O)cc1 |r| Show InChI InChI=1S/C26H28N2O3/c1-3-4-5-8-20-11-13-21(14-12-20)18-23(26(30)31)27-25(29)15-16-28-19(2)17-22-9-6-7-10-24(22)28/h6-7,9-14,17,23H,3-4,15-16,18H2,1-2H3,(H,27,29)(H,30,31)/t23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Binding affinity to human IL-2 measured over 30 mins by fluorescence polarization assay |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data