

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234829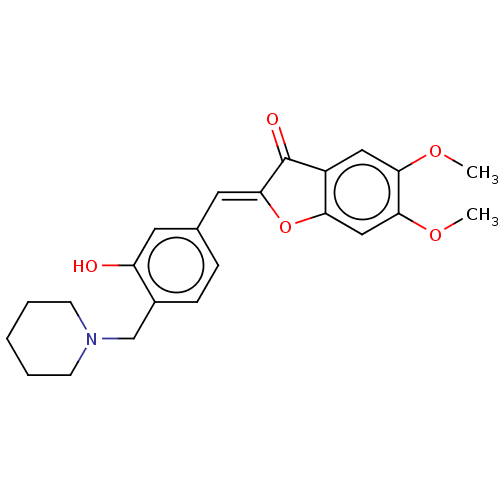 (CHEMBL4100760) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Mixed-type inhibition of Electrophorus electricus AChE pretreated for 15 mins followed by varying levels of acetylthiocholine iodide substrate additi... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-A expressed in baculovirus infected BTI insect cells using kynuramine as substrate incubated for 30 mins by fluor... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50234829 (CHEMBL4100760) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE in rat brain cortex homogenates using acetylthiocholine iodide as substrate in presence of BuChE inhibitor tetraisopropyl pyrophos... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE in rat brain cortex homogenates using acetylthiocholine iodide as substrate in presence of BuChE inhibitor tetraisopropyl pyrophos... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234829 (CHEMBL4100760) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50234829 (CHEMBL4100760) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM10989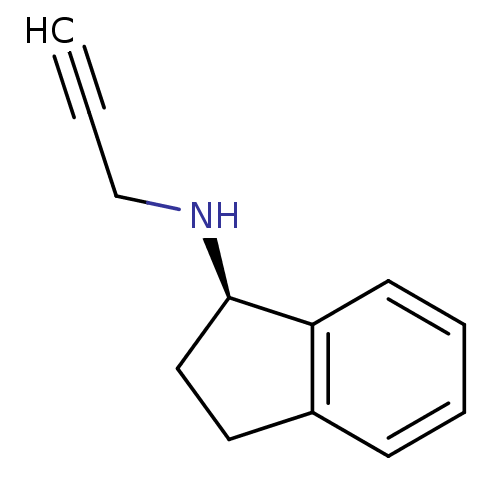 ((1R)-N-(prop-2-yn-1-yl)-2,3-dihydro-1H-inden-1-ami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B expressed in baculovirus infected BTI insect cells using kynuramine as substrate incubated for 30 mins by fluor... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50234814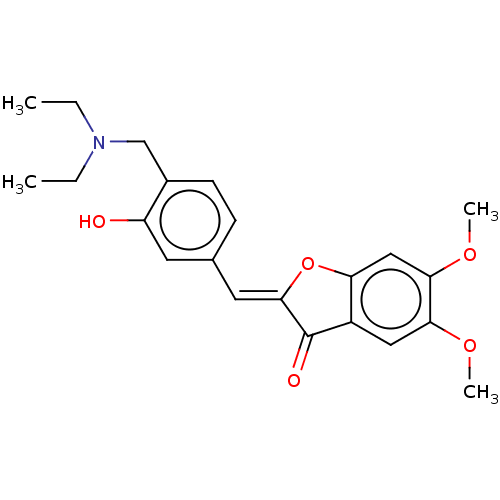 (CHEMBL4075571) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE in rat brain cortex homogenates using acetylthiocholine iodide as substrate in presence of BuChE inhibitor tetraisopropyl pyrophos... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50234823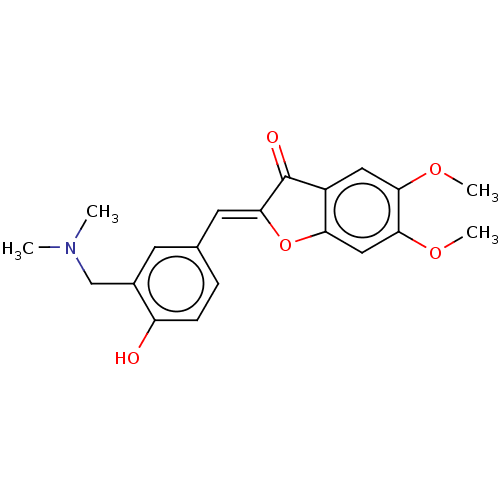 (CHEMBL4069184) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE in rat brain cortex homogenates using acetylthiocholine iodide as substrate in presence of BuChE inhibitor tetraisopropyl pyrophos... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50234815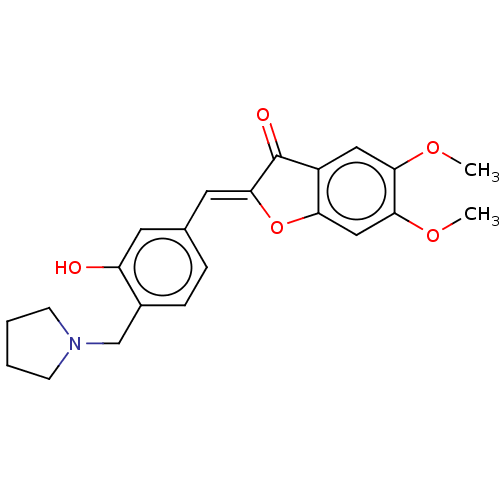 (CHEMBL4094805) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE in rat brain cortex homogenates using acetylthiocholine iodide as substrate in presence of BuChE inhibitor tetraisopropyl pyrophos... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234823 (CHEMBL4069184) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 411 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234814 (CHEMBL4075571) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 486 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50234823 (CHEMBL4069184) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50234814 (CHEMBL4075571) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50234815 (CHEMBL4094805) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM10989 ((1R)-N-(prop-2-yn-1-yl)-2,3-dihydro-1H-inden-1-ami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-A expressed in baculovirus infected BTI insect cells using kynuramine as substrate incubated for 30 mins by fluor... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM29136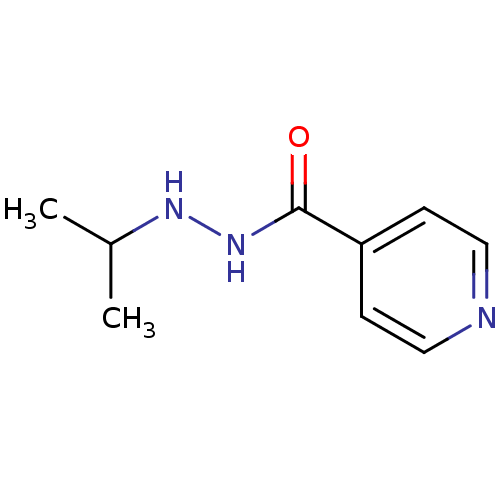 (CHEMBL92401 | Euphozid | Iprazid | Iproniazid) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B expressed in baculovirus infected BTI insect cells using kynuramine as substrate incubated for 30 mins by fluor... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM29136 (CHEMBL92401 | Euphozid | Iprazid | Iproniazid) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-A expressed in baculovirus infected BTI insect cells using kynuramine as substrate incubated for 30 mins by fluor... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234815 (CHEMBL4094805) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B expressed in baculovirus infected BTI insect cells using kynuramine as substrate incubated for 30 mins by fluor... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50234816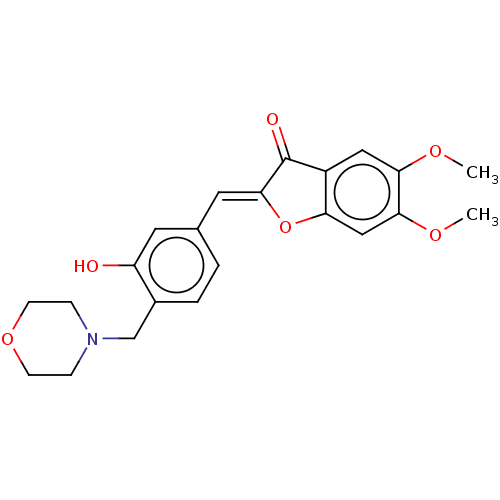 (CHEMBL4059887) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234821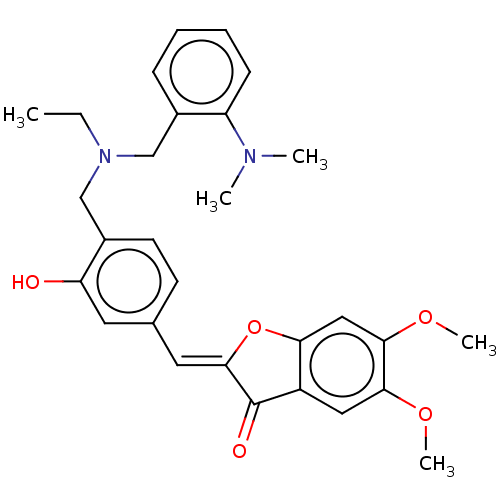 (CHEMBL4072914) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50234821 (CHEMBL4072914) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50234821 (CHEMBL4072914) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE in rat brain cortex homogenates using acetylthiocholine iodide as substrate in presence of BuChE inhibitor tetraisopropyl pyrophos... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50234816 (CHEMBL4059887) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE in rat brain cortex homogenates using acetylthiocholine iodide as substrate in presence of BuChE inhibitor tetraisopropyl pyrophos... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234813 (CHEMBL4090583) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234828 (CHEMBL4102562) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234818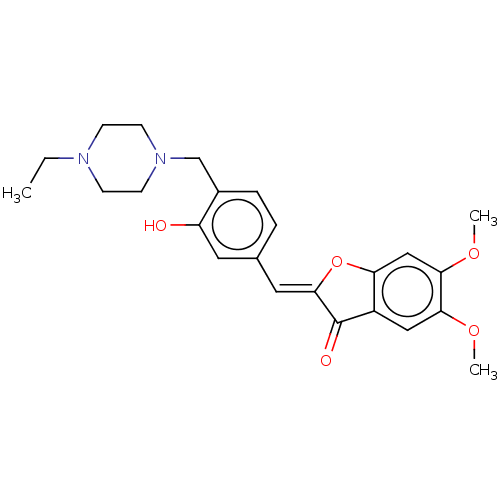 (CHEMBL4098196) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234817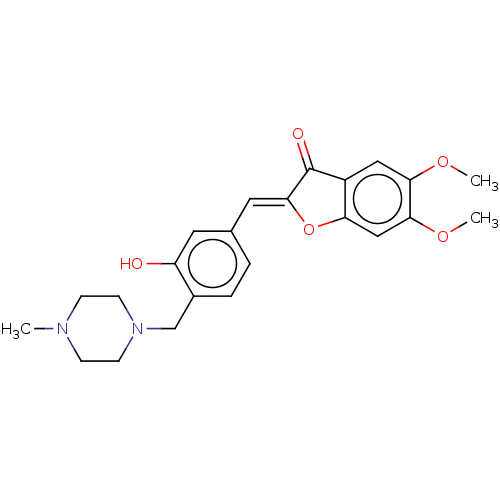 (CHEMBL4081805) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50234817 (CHEMBL4081805) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50234817 (CHEMBL4081805) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE in rat brain cortex homogenates using acetylthiocholine iodide as substrate in presence of BuChE inhibitor tetraisopropyl pyrophos... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234831 (CHEMBL4096430) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50234828 (CHEMBL4102562) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE in rat brain cortex homogenates using acetylthiocholine iodide as substrate in presence of BuChE inhibitor tetraisopropyl pyrophos... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234816 (CHEMBL4059887) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50234824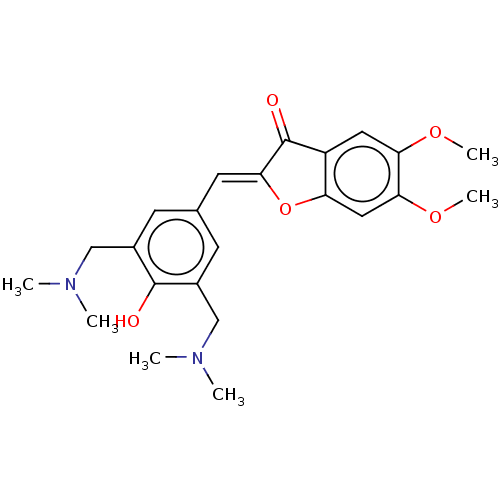 (CHEMBL4095726) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE in rat brain cortex homogenates using acetylthiocholine iodide as substrate in presence of BuChE inhibitor tetraisopropyl pyrophos... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50234818 (CHEMBL4098196) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE in rat brain cortex homogenates using acetylthiocholine iodide as substrate in presence of BuChE inhibitor tetraisopropyl pyrophos... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50234813 (CHEMBL4090583) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50234813 (CHEMBL4090583) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE in rat brain cortex homogenates using acetylthiocholine iodide as substrate in presence of BuChE inhibitor tetraisopropyl pyrophos... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50234828 (CHEMBL4102562) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50234831 (CHEMBL4096430) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE in rat brain cortex homogenates using acetylthiocholine iodide as substrate in presence of BuChE inhibitor tetraisopropyl pyrophos... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234819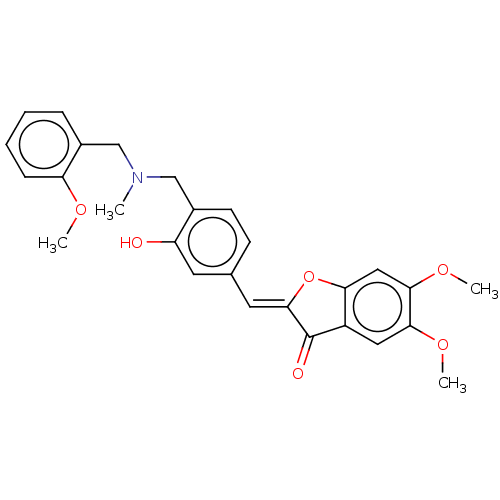 (CHEMBL4076740) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50234818 (CHEMBL4098196) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234825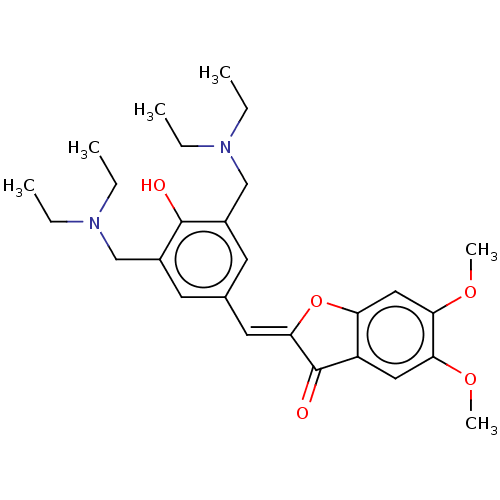 (CHEMBL4068377) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234820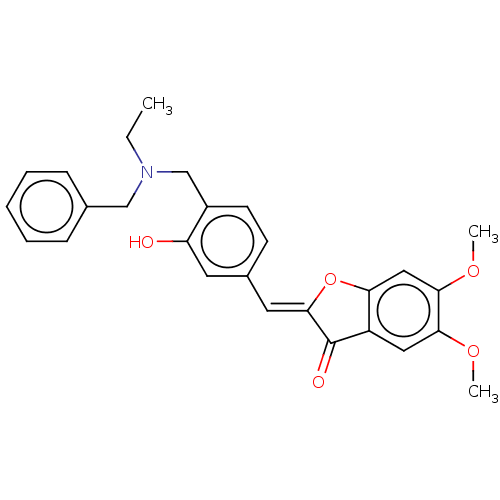 (CHEMBL4064330) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234827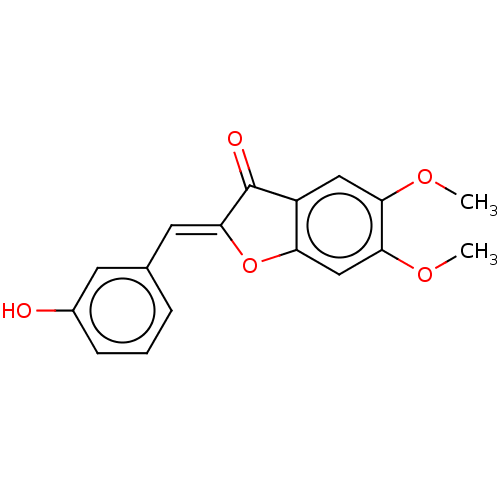 (CHEMBL4075780) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234830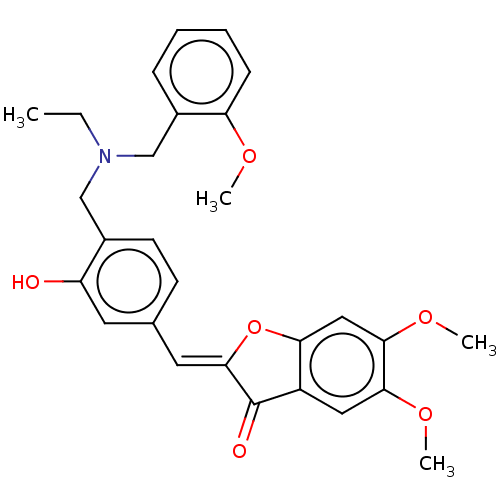 (CHEMBL4092053) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of BuChE in rat serum using butyrylthiocholine as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50234825 (CHEMBL4068377) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE in rat brain cortex homogenates using acetylthiocholine iodide as substrate in presence of BuChE inhibitor tetraisopropyl pyrophos... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50234830 (CHEMBL4092053) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE in rat brain cortex homogenates using acetylthiocholine iodide as substrate in presence of BuChE inhibitor tetraisopropyl pyrophos... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234824 (CHEMBL4095726) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234826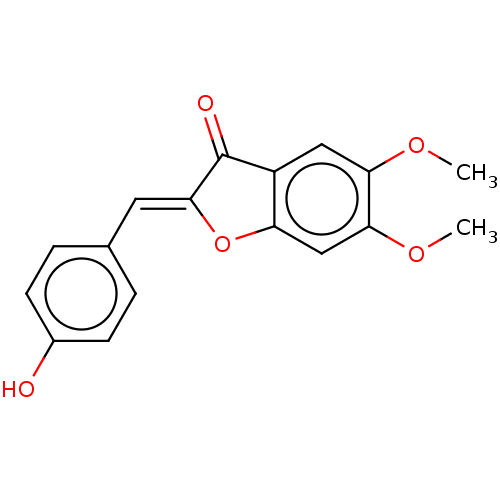 (CHEMBL4063760) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50234819 (CHEMBL4076740) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE in rat brain cortex homogenates using acetylthiocholine iodide as substrate in presence of BuChE inhibitor tetraisopropyl pyrophos... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50234820 (CHEMBL4064330) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE in rat brain cortex homogenates using acetylthiocholine iodide as substrate in presence of BuChE inhibitor tetraisopropyl pyrophos... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50234827 (CHEMBL4075780) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.31E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE in rat brain cortex homogenates using acetylthiocholine iodide as substrate in presence of BuChE inhibitor tetraisopropyl pyrophos... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50234822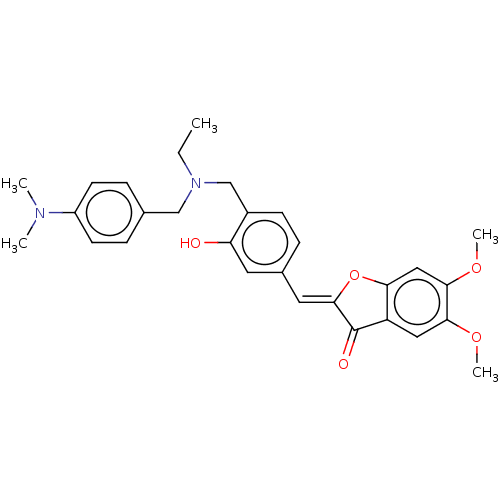 (CHEMBL4099799) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.88E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE in rat brain cortex homogenates using acetylthiocholine iodide as substrate in presence of BuChE inhibitor tetraisopropyl pyrophos... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50234826 (CHEMBL4063760) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.97E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE in rat brain cortex homogenates using acetylthiocholine iodide as substrate in presence of BuChE inhibitor tetraisopropyl pyrophos... | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234822 (CHEMBL4099799) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.31E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's method | Eur J Med Chem 126: 762-775 (2017) Article DOI: 10.1016/j.ejmech.2016.12.009 BindingDB Entry DOI: 10.7270/Q2NG4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||