 Found 20 hits of Enzyme Inhibition Constant Data
Found 20 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236280
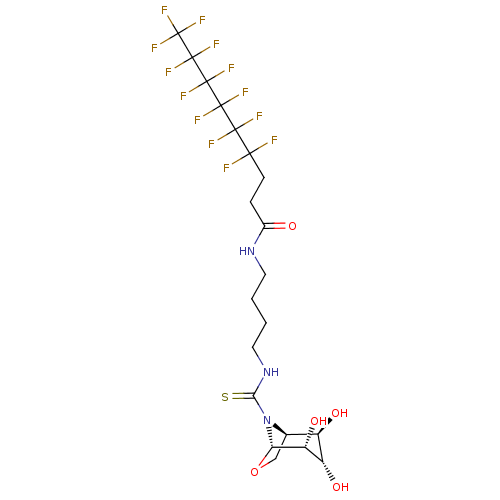
(CHEMBL4100971)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C20H24F13N3O5S/c21-15(22,16(23,24)17(25,26)18(27,28)19(29,30)20(31,32)33)4-3-9(37)34-5-1-2-6-35-14(42)36-8-7-41-13(36)12(40)11(39)10(8)38/h8,10-13,38-40H,1-7H2,(H,34,37)(H,35,42)/t8-,10-,11+,12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human whole blood Prostaglandin G/H synthase 2 |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236278
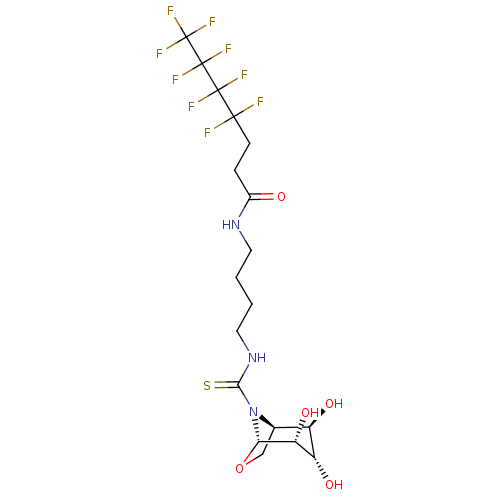
(CHEMBL4077472)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C18H24F9N3O5S/c19-15(20,16(21,22)17(23,24)18(25,26)27)4-3-9(31)28-5-1-2-6-29-14(36)30-8-7-35-13(30)12(34)11(33)10(8)32/h8,10-13,32-34H,1-7H2,(H,28,31)(H,29,36)/t8-,10-,11+,12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 7 in presence of bet... |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236279

(CHEMBL4093241)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCCCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C25H34F13N3O5S/c26-20(27,21(28,29)22(30,31)23(32,33)24(34,35)25(36,37)38)9-8-14(42)39-10-6-4-2-1-3-5-7-11-40-19(47)41-13-12-46-18(41)17(45)16(44)15(13)43/h13,15-18,43-45H,1-12H2,(H,39,42)(H,40,47)/t13-,15-,16+,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 7 in presence of bet... |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236281

(CHEMBL4076141)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCCCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C23H34F9N3O5S/c24-20(25,21(26,27)22(28,29)23(30,31)32)9-8-14(36)33-10-6-4-2-1-3-5-7-11-34-19(41)35-13-12-40-18(35)17(39)16(38)15(13)37/h13,15-18,37-39H,1-12H2,(H,33,36)(H,34,41)/t13-,15-,16+,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 7 in presence of bet... |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236280

(CHEMBL4100971)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C20H24F13N3O5S/c21-15(22,16(23,24)17(25,26)18(27,28)19(29,30)20(31,32)33)4-3-9(37)34-5-1-2-6-35-14(42)36-8-7-41-13(36)12(40)11(39)10(8)38/h8,10-13,38-40H,1-7H2,(H,34,37)(H,35,42)/t8-,10-,11+,12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human whole blood Prostaglandin G/H synthase 1 |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236279

(CHEMBL4093241)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCCCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C25H34F13N3O5S/c26-20(27,21(28,29)22(30,31)23(32,33)24(34,35)25(36,37)38)9-8-14(42)39-10-6-4-2-1-3-5-7-11-40-19(47)41-13-12-46-18(41)17(45)16(44)15(13)43/h13,15-18,43-45H,1-12H2,(H,39,42)(H,40,47)/t13-,15-,16+,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 7 by luminescence sp... |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236278

(CHEMBL4077472)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C18H24F9N3O5S/c19-15(20,16(21,22)17(23,24)18(25,26)27)4-3-9(31)28-5-1-2-6-29-14(36)30-8-7-35-13(30)12(34)11(33)10(8)32/h8,10-13,32-34H,1-7H2,(H,28,31)(H,29,36)/t8-,10-,11+,12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 5 in presence of bet... |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236279

(CHEMBL4093241)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCCCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C25H34F13N3O5S/c26-20(27,21(28,29)22(30,31)23(32,33)24(34,35)25(36,37)38)9-8-14(42)39-10-6-4-2-1-3-5-7-11-40-19(47)41-13-12-46-18(41)17(45)16(44)15(13)43/h13,15-18,43-45H,1-12H2,(H,39,42)(H,40,47)/t13-,15-,16+,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human whole blood Prostaglandin G/H synthase 2 |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236281

(CHEMBL4076141)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCCCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C23H34F9N3O5S/c24-20(25,21(26,27)22(28,29)23(30,31)32)9-8-14(36)33-10-6-4-2-1-3-5-7-11-34-19(41)35-13-12-40-18(35)17(39)16(38)15(13)37/h13,15-18,37-39H,1-12H2,(H,33,36)(H,34,41)/t13-,15-,16+,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 5 in presence of bet... |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236281

(CHEMBL4076141)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCCCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C23H34F9N3O5S/c24-20(25,21(26,27)22(28,29)23(30,31)32)9-8-14(36)33-10-6-4-2-1-3-5-7-11-34-19(41)35-13-12-40-18(35)17(39)16(38)15(13)37/h13,15-18,37-39H,1-12H2,(H,33,36)(H,34,41)/t13-,15-,16+,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human whole blood Prostaglandin G/H synthase 1 |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236280

(CHEMBL4100971)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C20H24F13N3O5S/c21-15(22,16(23,24)17(25,26)18(27,28)19(29,30)20(31,32)33)4-3-9(37)34-5-1-2-6-35-14(42)36-8-7-41-13(36)12(40)11(39)10(8)38/h8,10-13,38-40H,1-7H2,(H,34,37)(H,35,42)/t8-,10-,11+,12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 7 by luminescence sp... |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236278

(CHEMBL4077472)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C18H24F9N3O5S/c19-15(20,16(21,22)17(23,24)18(25,26)27)4-3-9(31)28-5-1-2-6-29-14(36)30-8-7-35-13(30)12(34)11(33)10(8)32/h8,10-13,32-34H,1-7H2,(H,28,31)(H,29,36)/t8-,10-,11+,12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 5 by luminescence sp... |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236279

(CHEMBL4093241)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCCCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C25H34F13N3O5S/c26-20(27,21(28,29)22(30,31)23(32,33)24(34,35)25(36,37)38)9-8-14(42)39-10-6-4-2-1-3-5-7-11-40-19(47)41-13-12-46-18(41)17(45)16(44)15(13)43/h13,15-18,43-45H,1-12H2,(H,39,42)(H,40,47)/t13-,15-,16+,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 5 by luminescence sp... |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236278

(CHEMBL4077472)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C18H24F9N3O5S/c19-15(20,16(21,22)17(23,24)18(25,26)27)4-3-9(31)28-5-1-2-6-29-14(36)30-8-7-35-13(30)12(34)11(33)10(8)32/h8,10-13,32-34H,1-7H2,(H,28,31)(H,29,36)/t8-,10-,11+,12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 7 by luminescence sp... |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236281

(CHEMBL4076141)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCCCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C23H34F9N3O5S/c24-20(25,21(26,27)22(28,29)23(30,31)32)9-8-14(36)33-10-6-4-2-1-3-5-7-11-34-19(41)35-13-12-40-18(35)17(39)16(38)15(13)37/h13,15-18,37-39H,1-12H2,(H,33,36)(H,34,41)/t13-,15-,16+,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 5 by luminescence sp... |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50236280

(CHEMBL4100971)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C20H24F13N3O5S/c21-15(22,16(23,24)17(25,26)18(27,28)19(29,30)20(31,32)33)4-3-9(37)34-5-1-2-6-35-14(42)36-8-7-41-13(36)12(40)11(39)10(8)38/h8,10-13,38-40H,1-7H2,(H,34,37)(H,35,42)/t8-,10-,11+,12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human whole blood Prostaglandin G/H synthase 1 |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase
(Canavalia ensiformis) | BDBM50236280

(CHEMBL4100971)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C20H24F13N3O5S/c21-15(22,16(23,24)17(25,26)18(27,28)19(29,30)20(31,32)33)4-3-9(37)34-5-1-2-6-35-14(42)36-8-7-41-13(36)12(40)11(39)10(8)38/h8,10-13,38-40H,1-7H2,(H,34,37)(H,35,42)/t8-,10-,11+,12-,13+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
Inhibition of jack bean alpha-mannosidase using beta-D-glycopyranoside after 10 to 30 mins by spectrophotometry |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase
(Canavalia ensiformis) | BDBM50236278

(CHEMBL4077472)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C18H24F9N3O5S/c19-15(20,16(21,22)17(23,24)18(25,26)27)4-3-9(31)28-5-1-2-6-29-14(36)30-8-7-35-13(30)12(34)11(33)10(8)32/h8,10-13,32-34H,1-7H2,(H,28,31)(H,29,36)/t8-,10-,11+,12-,13+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
Binding affinity towards dopamine transporter using [3H]- mazindol as radioligand in rat striatal membranes |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Alpha-galactosidase
(Coffea arabica (Coffee beans)) | BDBM50236280

(CHEMBL4100971)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C20H24F13N3O5S/c21-15(22,16(23,24)17(25,26)18(27,28)19(29,30)20(31,32)33)4-3-9(37)34-5-1-2-6-35-14(42)36-8-7-41-13(36)12(40)11(39)10(8)38/h8,10-13,38-40H,1-7H2,(H,34,37)(H,35,42)/t8-,10-,11+,12-,13+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
Inhibition of green coffee bean alpha-galactosidase using beta-D-glycopyranoside after 10 to 30 mins by spectrophotometry |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |
Alpha-galactosidase
(Coffea arabica (Coffee beans)) | BDBM50236278

(CHEMBL4077472)Show SMILES [H][C@@]12CO[C@@]([H])([C@H](O)[C@@H](O)[C@@H]1O)N2C(=S)NCCCCNC(=O)CCC(F)(F)C(F)(F)C(F)(F)C(F)(F)F |r,TLB:7:6:2.3:12,THB:11:10:2.3:12| Show InChI InChI=1S/C18H24F9N3O5S/c19-15(20,16(21,22)17(23,24)18(25,26)27)4-3-9(31)28-5-1-2-6-29-14(36)30-8-7-35-13(30)12(34)11(33)10(8)32/h8,10-13,32-34H,1-7H2,(H,28,31)(H,29,36)/t8-,10-,11+,12-,13+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla
Curated by ChEMBL
| Assay Description
Inhibition of green coffee bean alpha-galactosidase using beta-D-glycopyranoside after 10 to 30 mins by spectrophotometry |
J Med Chem 60: 1829-1842 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01550
BindingDB Entry DOI: 10.7270/Q2K939S5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data