

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Anopheles gambiae) | BDBM50252257 (CHEMBL4092186) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of recombinant full length Anopheles gambiae AChE1 expressed in baculovirus-infected Sf9 insect cells using acetylthiocholine iodide as su... | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Anopheles gambiae) | BDBM50252257 (CHEMBL4092186) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of AChE1 in Anopheles gambiae head extract using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Anopheles gambiae) | BDBM50252257 (CHEMBL4092186) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of AChE1 in Anopheles gambiae head extract using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Anopheles gambiae) | BDBM50252266 (CHEMBL1340607) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of AChE1 in Anopheles gambiae body extract using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Anopheles gambiae) | BDBM50252256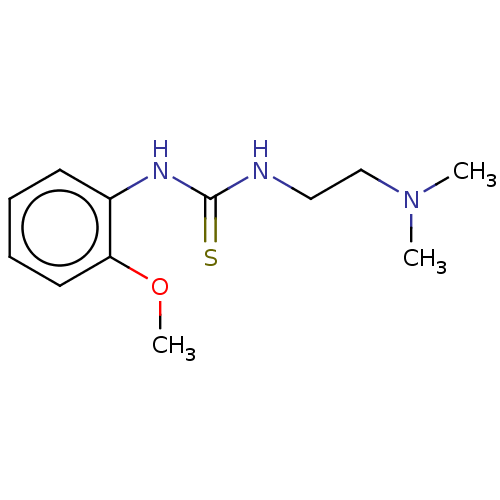 (CHEMBL4064806) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of recombinant full length Anopheles gambiae AChE1 expressed in baculovirus-infected Sf9 insect cells using acetylthiocholine iodide as su... | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Anopheles gambiae) | BDBM50252256 (CHEMBL4064806) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of AChE1 in Anopheles gambiae body extract using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Anopheles gambiae) | BDBM50252256 (CHEMBL4064806) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of AChE1 in Anopheles gambiae body extract using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Anopheles gambiae) | BDBM50252272 (CHEMBL4079963) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of AChE1 in Anopheles gambiae body extract using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Anopheles gambiae) | BDBM50252254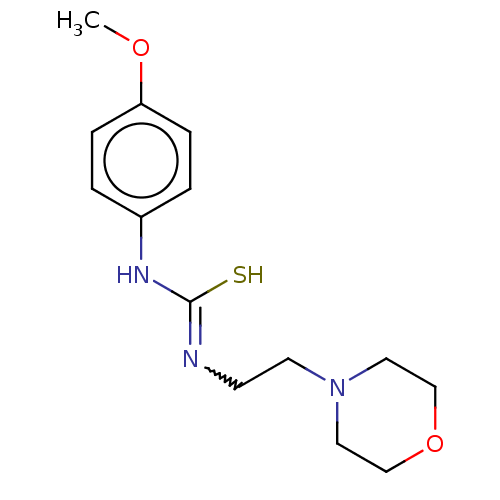 (CHEMBL1543212) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of AChE1 in Anopheles gambiae body extract using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50252266 (CHEMBL1340607) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293F cells using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50252257 (CHEMBL4092186) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293F cells using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Anopheles gambiae) | BDBM50252263 (CHEMBL4100253) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of AChE1 in Anopheles gambiae body extract using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Anopheles gambiae) | BDBM50252258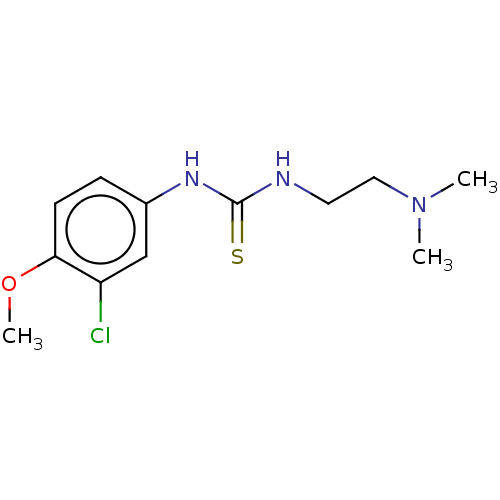 (CHEMBL4075136) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of AChE1 in Anopheles gambiae body extract using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Anopheles gambiae) | BDBM50252264 (CHEMBL4073401) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of AChE1 in Anopheles gambiae body extract using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Anopheles gambiae) | BDBM50252268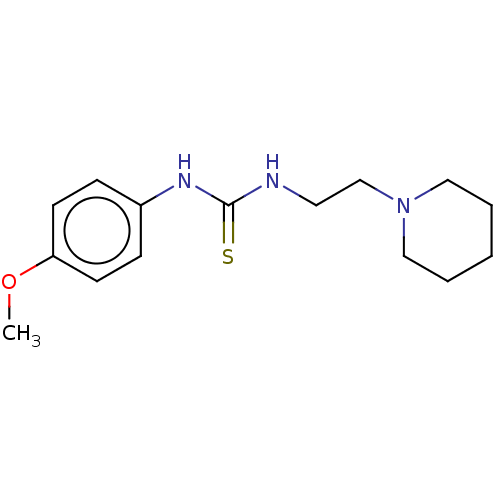 (CHEMBL4099934) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of AChE1 in Anopheles gambiae body extract using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50252264 (CHEMBL4073401) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293F cells using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50252258 (CHEMBL4075136) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293F cells using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Anopheles gambiae) | BDBM50252255 (CHEMBL1416246) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of AChE1 in Anopheles gambiae body extract using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Anopheles gambiae) | BDBM50171021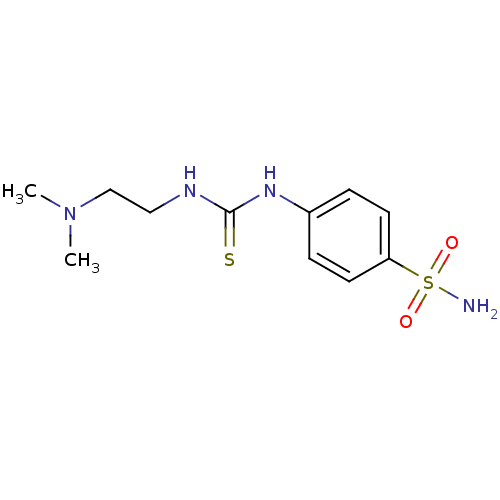 (4-[3-(2-Dimethylamino-ethyl)-thioureido]-benzenesu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of AChE1 in Anopheles gambiae body extract using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50252272 (CHEMBL4079963) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293F cells using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Anopheles gambiae) | BDBM50252260 (CHEMBL1301463) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of AChE1 in Anopheles gambiae body extract using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50252268 (CHEMBL4099934) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293F cells using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50252256 (CHEMBL4064806) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293F cells using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50252260 (CHEMBL1301463) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293F cells using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Anopheles gambiae) | BDBM50252269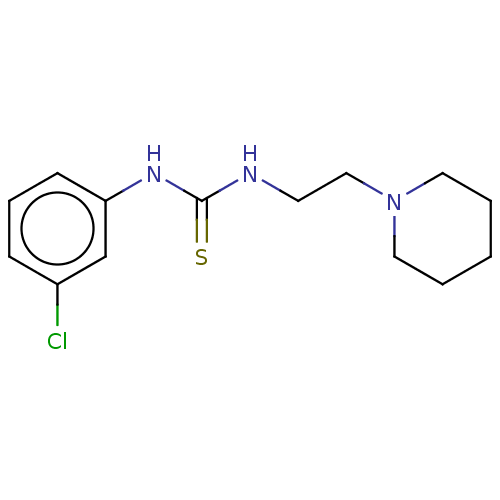 (CHEMBL4071394) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of AChE1 in Anopheles gambiae body extract using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50252269 (CHEMBL4071394) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293F cells using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Anopheles gambiae) | BDBM50252267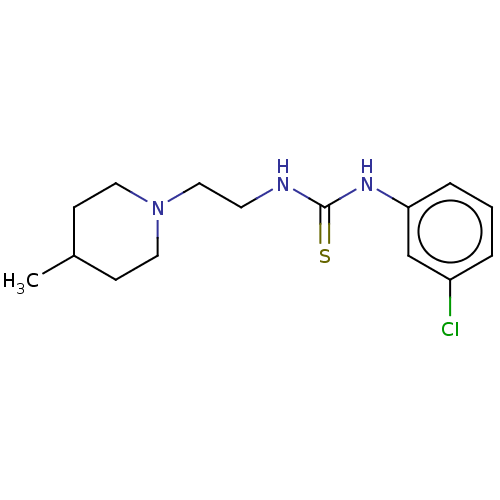 (CHEMBL4081932) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of AChE1 in Anopheles gambiae body extract using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50252263 (CHEMBL4100253) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293F cells using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50171021 (4-[3-(2-Dimethylamino-ethyl)-thioureido]-benzenesu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293F cells using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Anopheles gambiae) | BDBM50252271 (CHEMBL4095547) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of AChE1 in Anopheles gambiae body extract using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Anopheles gambiae) | BDBM50252265 (CHEMBL1311140) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of AChE1 in Anopheles gambiae body extract using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50252262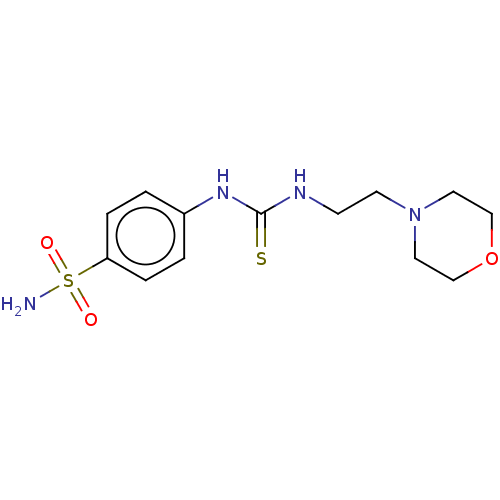 (CHEMBL4092142) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293F cells using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50252259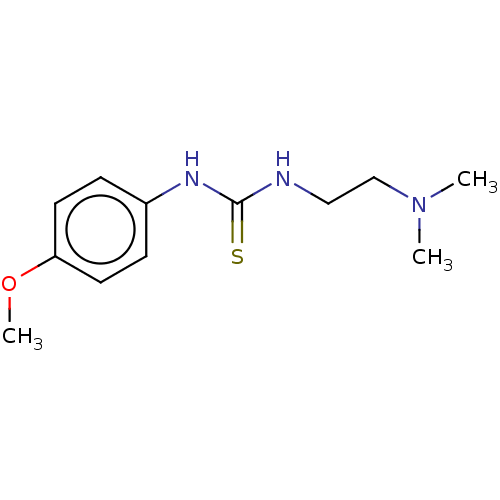 (CHEMBL4087841) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293F cells using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Anopheles gambiae) | BDBM50252270 (CHEMBL1482486) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of AChE1 in Anopheles gambiae body extract using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Anopheles gambiae) | BDBM50252259 (CHEMBL4087841) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of AChE1 in Anopheles gambiae body extract using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50252265 (CHEMBL1311140) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293F cells using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50252270 (CHEMBL1482486) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293F cells using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Anopheles gambiae) | BDBM50252261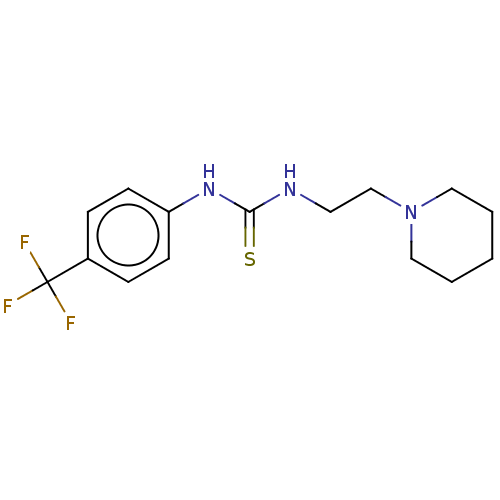 (CHEMBL4104206) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of AChE1 in Anopheles gambiae body extract using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50252267 (CHEMBL4081932) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293F cells using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Anopheles gambiae) | BDBM50252262 (CHEMBL4092142) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of AChE1 in Anopheles gambiae body extract using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50252261 (CHEMBL4104206) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293F cells using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50252271 (CHEMBL4095547) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293F cells using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50252255 (CHEMBL1416246) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293F cells using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50252254 (CHEMBL1543212) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293F cells using acetylthiocholine iodide as substrate measured over 60 secs by Ellman's method | Eur J Med Chem 134: 415-427 (2017) Article DOI: 10.1016/j.ejmech.2017.03.050 BindingDB Entry DOI: 10.7270/Q23X893Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||