 Found 33 hits of Enzyme Inhibition Constant Data
Found 33 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of fibrinogen receptor |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
Inhibition of ADP-induced platelet aggregation in human platelet-rich plasma |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50241719
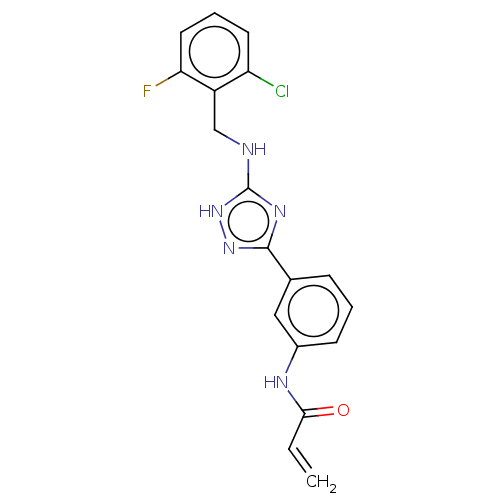
(CHEMBL4101977)Show SMILES Fc1cccc(Cl)c1CNc1nc(n[nH]1)-c1cccc(NC(=O)C=C)c1 Show InChI InChI=1S/C18H15ClFN5O/c1-2-16(26)22-12-6-3-5-11(9-12)17-23-18(25-24-17)21-10-13-14(19)7-4-8-15(13)20/h2-9H,1,10H2,(H,22,26)(H2,21,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
Inhibition of ADP-induced platelet aggregation in human platelet-rich plasma |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
Inhibition of human TEC using poly[Glu:Tyr] as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition by filter binding method |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of fibrinogen receptor |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50241719

(CHEMBL4101977)Show SMILES Fc1cccc(Cl)c1CNc1nc(n[nH]1)-c1cccc(NC(=O)C=C)c1 Show InChI InChI=1S/C18H15ClFN5O/c1-2-16(26)22-12-6-3-5-11(9-12)17-23-18(25-24-17)21-10-13-14(19)7-4-8-15(13)20/h2-9H,1,10H2,(H,22,26)(H2,21,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
Inhibition of ADP-induced platelet aggregation in human platelet-rich plasma |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50241732
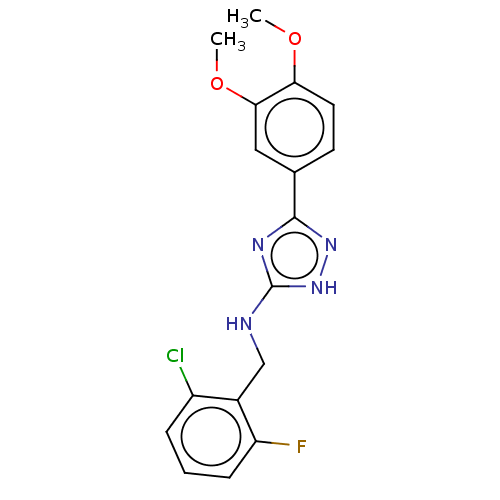
(CHEMBL4091764)Show InChI InChI=1S/C17H16ClFN4O2/c1-24-14-7-6-10(8-15(14)25-2)16-21-17(23-22-16)20-9-11-12(18)4-3-5-13(11)19/h3-8H,9H2,1-2H3,(H2,20,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
Inhibition of ADP-induced platelet aggregation in human platelet-rich plasma |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50241719

(CHEMBL4101977)Show SMILES Fc1cccc(Cl)c1CNc1nc(n[nH]1)-c1cccc(NC(=O)C=C)c1 Show InChI InChI=1S/C18H15ClFN5O/c1-2-16(26)22-12-6-3-5-11(9-12)17-23-18(25-24-17)21-10-13-14(19)7-4-8-15(13)20/h2-9H,1,10H2,(H,22,26)(H2,21,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
Inhibition of ADP-induced platelet aggregation in human platelet-rich plasma |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50241731
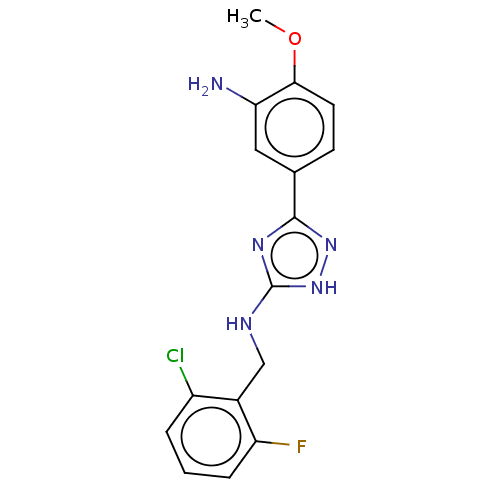
(CHEMBL4064000)Show InChI InChI=1S/C16H15ClFN5O/c1-24-14-6-5-9(7-13(14)19)15-21-16(23-22-15)20-8-10-11(17)3-2-4-12(10)18/h2-7H,8,19H2,1H3,(H2,20,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged full length recombinant human LCK expressed in baculovirus expression system using TK-substrate-biotin after 40 mins by HTRF... |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50241733
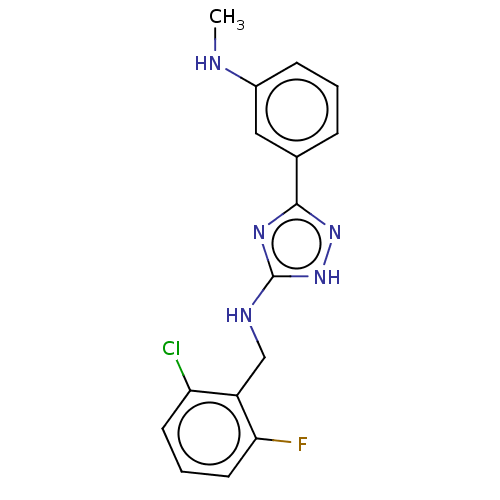
(CHEMBL4073371)Show InChI InChI=1S/C16H15ClFN5/c1-19-11-5-2-4-10(8-11)15-21-16(23-22-15)20-9-12-13(17)6-3-7-14(12)18/h2-8,19H,9H2,1H3,(H2,20,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged full length recombinant human LCK expressed in baculovirus expression system using TK-substrate-biotin after 40 mins by HTRF... |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50241730
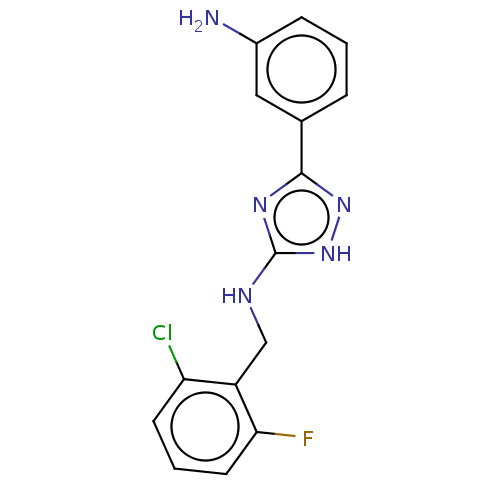
(CHEMBL4100228)Show InChI InChI=1S/C15H13ClFN5/c16-12-5-2-6-13(17)11(12)8-19-15-20-14(21-22-15)9-3-1-4-10(18)7-9/h1-7H,8,18H2,(H2,19,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
Inhibition of ADP-induced platelet aggregation in human platelet-rich plasma |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50241732

(CHEMBL4091764)Show InChI InChI=1S/C17H16ClFN4O2/c1-24-14-7-6-10(8-15(14)25-2)16-21-17(23-22-16)20-9-11-12(18)4-3-5-13(11)19/h3-8H,9H2,1-2H3,(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of fibrinogen receptor |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50241733

(CHEMBL4073371)Show InChI InChI=1S/C16H15ClFN5/c1-19-11-5-2-4-10(8-11)15-21-16(23-22-15)20-9-12-13(17)6-3-7-14(12)18/h2-8,19H,9H2,1H3,(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 271 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
Inhibition of ADP-induced platelet aggregation in human platelet-rich plasma |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50241719

(CHEMBL4101977)Show SMILES Fc1cccc(Cl)c1CNc1nc(n[nH]1)-c1cccc(NC(=O)C=C)c1 Show InChI InChI=1S/C18H15ClFN5O/c1-2-16(26)22-12-6-3-5-11(9-12)17-23-18(25-24-17)21-10-13-14(19)7-4-8-15(13)20/h2-9H,1,10H2,(H,22,26)(H2,21,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 306 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
Inhibition of human TEC using poly[Glu:Tyr] as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition by filter binding method |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50241731

(CHEMBL4064000)Show InChI InChI=1S/C16H15ClFN5O/c1-24-14-6-5-9(7-13(14)19)15-21-16(23-22-15)20-8-10-11(17)3-2-4-12(10)18/h2-7H,8,19H2,1H3,(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 334 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of fibrinogen receptor |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50241729

(CHEMBL4079354)Show InChI InChI=1S/C15H12ClFN4O/c16-12-5-2-6-13(17)11(12)8-18-15-19-14(20-21-15)9-3-1-4-10(22)7-9/h1-7,22H,8H2,(H2,18,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 337 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
Inhibition of ADP-induced platelet aggregation in human platelet-rich plasma |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50241728

(CHEMBL4103618)Show InChI InChI=1S/C15H12ClFN4/c16-12-7-4-8-13(17)11(12)9-18-15-19-14(20-21-15)10-5-2-1-3-6-10/h1-8H,9H2,(H2,18,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 468 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged full length recombinant human LCK expressed in baculovirus expression system using TK-substrate-biotin after 40 mins by HTRF... |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50241718
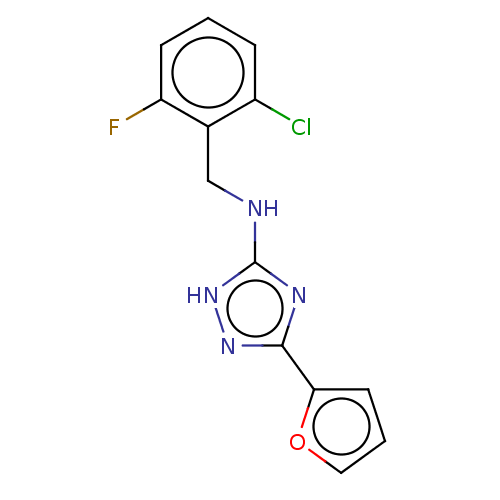
(CHEMBL1354522)Show InChI InChI=1S/C13H10ClFN4O/c14-9-3-1-4-10(15)8(9)7-16-13-17-12(18-19-13)11-5-2-6-20-11/h1-6H,7H2,(H2,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human LYN using TK-substrate-biotin after 40 mins by HTRF assay |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50241730

(CHEMBL4100228)Show InChI InChI=1S/C15H13ClFN5/c16-12-5-2-6-13(17)11(12)8-19-15-20-14(21-22-15)9-3-1-4-10(18)7-9/h1-7H,8,18H2,(H2,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 526 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
Inhibition of Fibrinogen binding to Fibrinogen receptor |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50241718

(CHEMBL1354522)Show InChI InChI=1S/C13H10ClFN4O/c14-9-3-1-4-10(15)8(9)7-16-13-17-12(18-19-13)11-5-2-6-20-11/h1-6H,7H2,(H2,16,17,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 531 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of fibrinogen receptor |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50241718

(CHEMBL1354522)Show InChI InChI=1S/C13H10ClFN4O/c14-9-3-1-4-10(15)8(9)7-16-13-17-12(18-19-13)11-5-2-6-20-11/h1-6H,7H2,(H2,16,17,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of fibrinogen receptor |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50241718

(CHEMBL1354522)Show InChI InChI=1S/C13H10ClFN4O/c14-9-3-1-4-10(15)8(9)7-16-13-17-12(18-19-13)11-5-2-6-20-11/h1-6H,7H2,(H2,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
Inhibition of ADP-induced platelet aggregation in human platelet-rich plasma |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50241729

(CHEMBL4079354)Show InChI InChI=1S/C15H12ClFN4O/c16-12-5-2-6-13(17)11(12)8-18-15-19-14(20-21-15)9-3-1-4-10(22)7-9/h1-7,22H,8H2,(H2,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of fibrinogen receptor |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50241728

(CHEMBL4103618)Show InChI InChI=1S/C15H12ClFN4/c16-12-7-4-8-13(17)11(12)9-18-15-19-14(20-21-15)10-5-2-1-3-6-10/h1-8H,9H2,(H2,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of fibrinogen receptor |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50241730

(CHEMBL4100228)Show InChI InChI=1S/C15H13ClFN5/c16-12-5-2-6-13(17)11(12)8-19-15-20-14(21-22-15)9-3-1-4-10(18)7-9/h1-7H,8,18H2,(H2,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
Inhibition of ADP-induced platelet aggregation in human platelet-rich plasma |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50241729

(CHEMBL4079354)Show InChI InChI=1S/C15H12ClFN4O/c16-12-5-2-6-13(17)11(12)8-18-15-19-14(20-21-15)9-3-1-4-10(22)7-9/h1-7,22H,8H2,(H2,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of fibrinogen receptor |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50241718

(CHEMBL1354522)Show InChI InChI=1S/C13H10ClFN4O/c14-9-3-1-4-10(15)8(9)7-16-13-17-12(18-19-13)11-5-2-6-20-11/h1-6H,7H2,(H2,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of fibrinogen receptor |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50241718

(CHEMBL1354522)Show InChI InChI=1S/C13H10ClFN4O/c14-9-3-1-4-10(15)8(9)7-16-13-17-12(18-19-13)11-5-2-6-20-11/h1-6H,7H2,(H2,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged full length recombinant human BMX expressed in baculovirus expression system using TK-substrate-biotin after 40 mins by HTRF... |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50241719

(CHEMBL4101977)Show SMILES Fc1cccc(Cl)c1CNc1nc(n[nH]1)-c1cccc(NC(=O)C=C)c1 Show InChI InChI=1S/C18H15ClFN5O/c1-2-16(26)22-12-6-3-5-11(9-12)17-23-18(25-24-17)21-10-13-14(19)7-4-8-15(13)20/h2-9H,1,10H2,(H,22,26)(H2,21,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of fibrinogen receptor |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50241728

(CHEMBL4103618)Show InChI InChI=1S/C15H12ClFN4/c16-12-7-4-8-13(17)11(12)9-18-15-19-14(20-21-15)10-5-2-1-3-6-10/h1-8H,9H2,(H2,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
Inhibition of ADP-induced platelet aggregation in human platelet-rich plasma |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50241733

(CHEMBL4073371)Show InChI InChI=1S/C16H15ClFN5/c1-19-11-5-2-4-10(8-11)15-21-16(23-22-15)20-9-12-13(17)6-3-7-14(12)18/h2-8,19H,9H2,1H3,(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of fibrinogen receptor |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50241731

(CHEMBL4064000)Show InChI InChI=1S/C16H15ClFN5O/c1-24-14-6-5-9(7-13(14)19)15-21-16(23-22-15)20-8-10-11(17)3-2-4-12(10)18/h2-7H,8,19H2,1H3,(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
Inhibition of ADP-induced platelet aggregation in human platelet-rich plasma |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50241732

(CHEMBL4091764)Show InChI InChI=1S/C17H16ClFN4O2/c1-24-14-7-6-10(8-15(14)25-2)16-21-17(23-22-16)20-9-11-12(18)4-3-5-13(11)19/h3-8H,9H2,1-2H3,(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of fibrinogen receptor |
J Med Chem 60: 8552-8564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01075
BindingDB Entry DOI: 10.7270/Q25X2C34 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data