 Found 64 hits of Enzyme Inhibition Constant Data
Found 64 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM310195
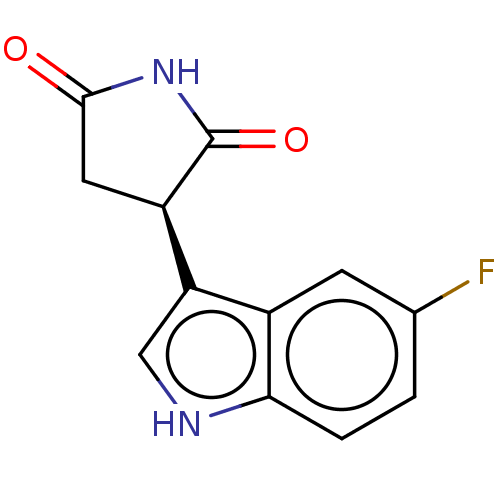
((-)-(R)-3-(5-fluoro-1H- indol-3-yl)pyrrolidine- 2,...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM309529

(3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM310195

((-)-(R)-3-(5-fluoro-1H- indol-3-yl)pyrrolidine- 2,...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using tryptophan as substrate after 22 mins by LC-MS/MS method |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM312073
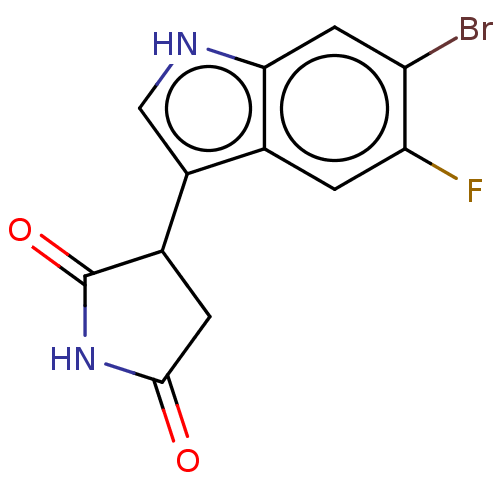
(3-(6-bromo-5-fluoro- 1H-indol-3-yl)- pyrrolidine-2...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM312076
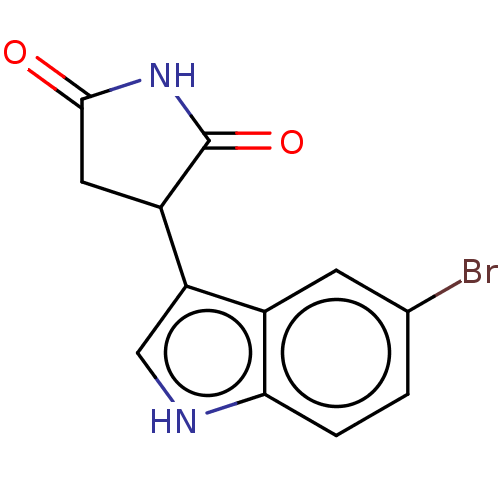
(3-(5-bromo-1H-indol-3- yl)pyrrolidine-2,5-dione | ...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM309529

(3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using tryptophan as substrate after 22 mins by LC-MS/MS method |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM312084
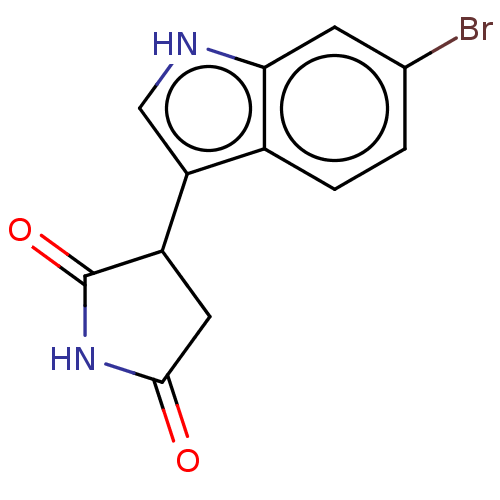
(3-(6-bromo-1H-indol-3- yl)pyrrolidine-2,5-dione | ...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM312072
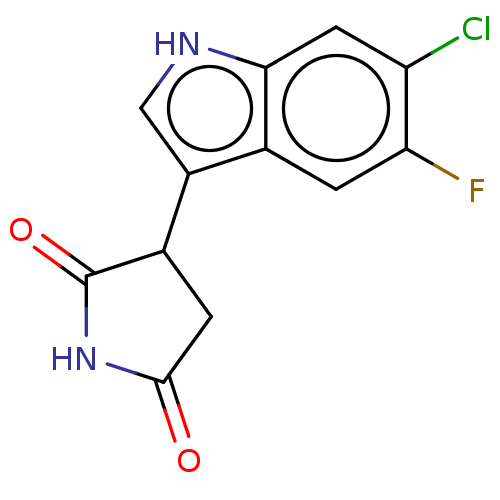
(3-(6-chloro-5-fluoro-1H- indol-3-yl)pyrrolidine- 2...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM389161
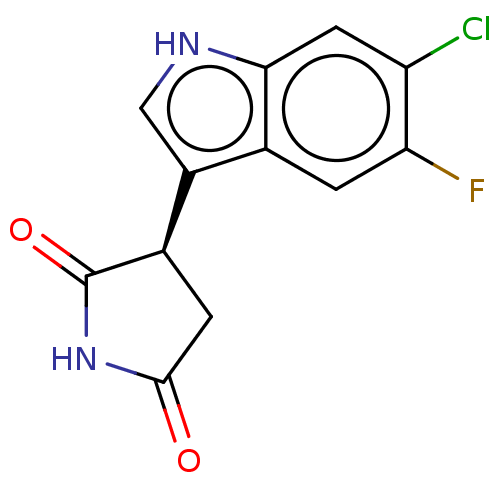
((R)-3-(6-chloro-5- fluoro-1H-indol-3-yl) pyrrolidi...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM312074
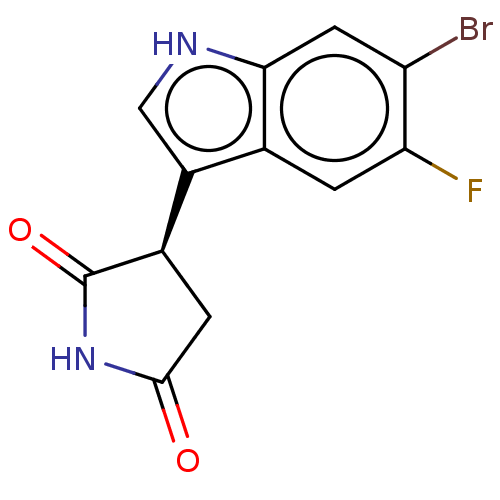
((R)-3-(6-bromo-5- fluoro-1H-indol-3-yl)- pyrrolidi...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM312069
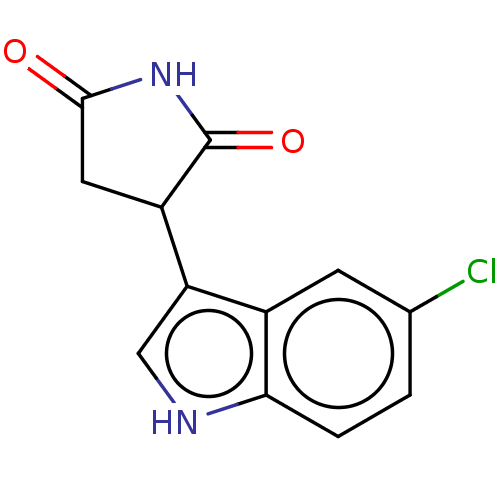
(3-(5-chloro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM310195

((-)-(R)-3-(5-fluoro-1H- indol-3-yl)pyrrolidine- 2,...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of IDO-1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine production after 16 to 24 hrs by PDMAB method |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM310195

((-)-(R)-3-(5-fluoro-1H- indol-3-yl)pyrrolidine- 2,...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of IDO-1 in IFN-gamma/LPS-stimulated human THP1 cells assessed as decrease in kynurenine production after 16 to 24 hrs by PDMAB method |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM389150

(3-(2,5-dioxopyrrolidin- 3-yl)-1H-indole-5- carboni...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM309529

(3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of mouse IDO-1 using tryptophan as substrate after 22 mins by LC-MS/MS method |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM389157
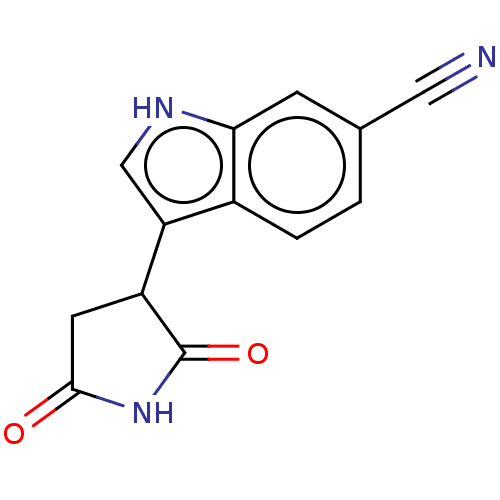
(3-(2,5-dioxopyrrolidin- 3-yl)-1H-indole-6- carboni...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM312088
(3-(6-fluoronaphthanlen- 1-yl)pyrrolidine-2,5- dion...)Show InChI InChI=1S/C14H10FNO2/c15-9-4-5-10-8(6-9)2-1-3-11(10)12-7-13(17)16-14(12)18/h1-6,12H,7H2,(H,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM309529

(3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of IDO-1 in IFN-gamma/LPS-stimulated human THP1 cells assessed as decrease in kynurenine production after 16 to 24 hrs by PDMAB method |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM312080
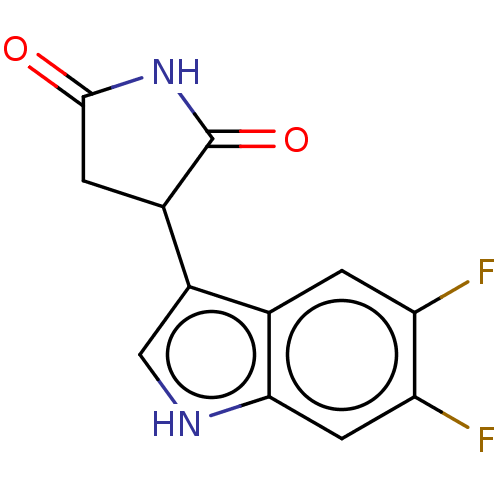
(3-(5,6-difluoro-1H- indol-3-yl)pyrrolidine- 2,5-di...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM309529

(3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of IDO-1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine production after 16 to 24 hrs by PDMAB method |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM312064
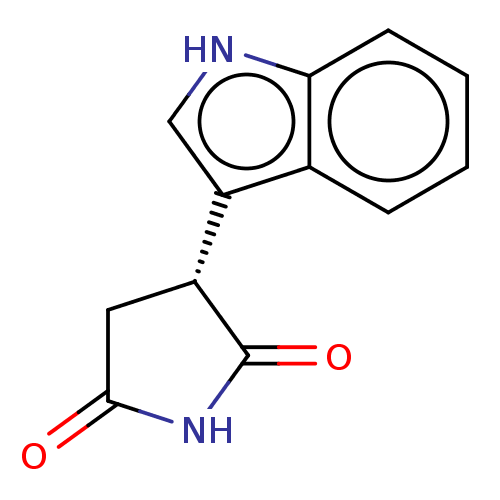
((-)-(R)-3-(1H-indol-3- yl)pyrrolidine-2,5-dione | ...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM312083
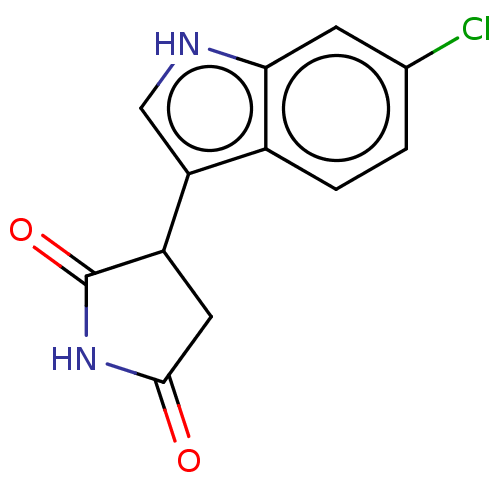
(3-(6-chloro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM312070
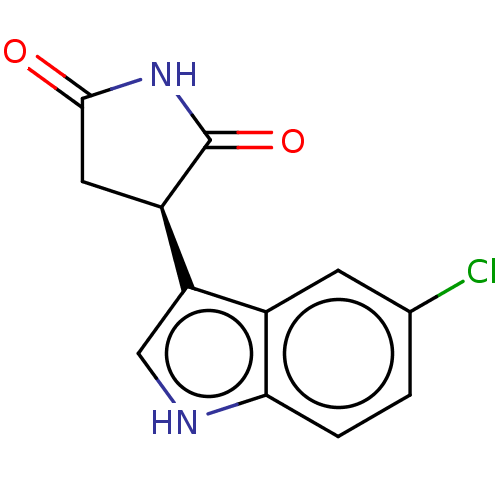
((-)-(R)-3-(5-chloro-1H- indol-3-yl)pyrrolidine- 2,...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM310195

((-)-(R)-3-(5-fluoro-1H- indol-3-yl)pyrrolidine- 2,...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of IDO-1 in IFN-gamma/LPS-stimulated human whole blood assessed as decrease in kynurenine production after 24 hrs by LC-MS/MS method |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM73346
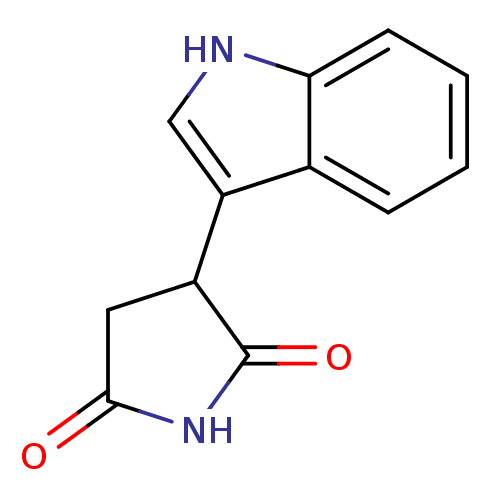
(3-(1H-indol-3-yl)-2,5-pyrrolidinedione | 3-(1H-ind...)Show InChI InChI=1S/C12H10N2O2/c15-11-5-8(12(16)14-11)9-6-13-10-4-2-1-3-7(9)10/h1-4,6,8,13H,5H2,(H,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM312082
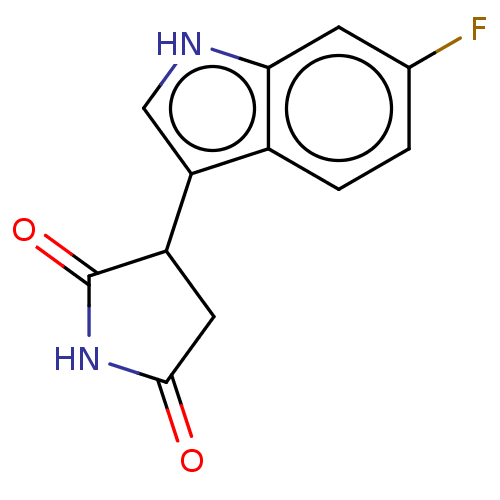
(3-(6-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM312089
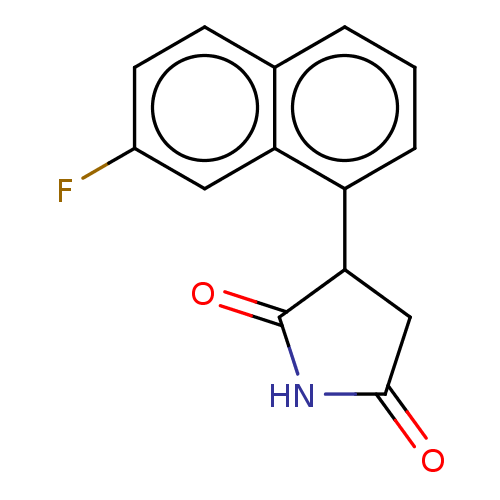
(3-(7-fluoronaphthanlen- 1-yl)pyrrolidine-2,5- dion...)Show InChI InChI=1S/C14H10FNO2/c15-9-5-4-8-2-1-3-10(11(8)6-9)12-7-13(17)16-14(12)18/h1-6,12H,7H2,(H,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM309529

(3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of IDO-1 in IFN-gamma/LPS-stimulated human whole blood assessed as decrease in kynurenine production after 24 hrs by LC-MS/MS method |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM310195

((-)-(R)-3-(5-fluoro-1H- indol-3-yl)pyrrolidine- 2,...) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of mouse IDO-1 using tryptophan as substrate after 22 mins by LC-MS/MS method |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM312075
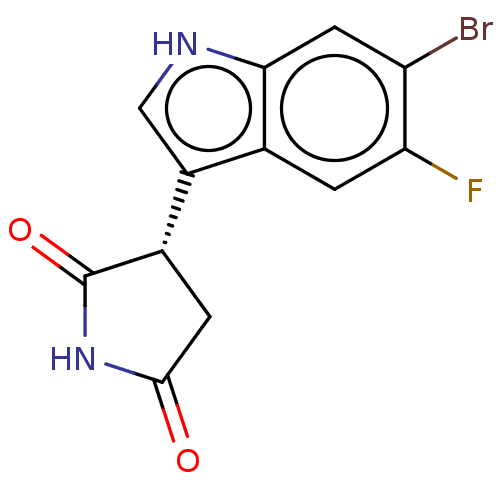
(US9603836, Compound 10a | US9949951, 10a)Show SMILES Fc1cc2c(c[nH]c2cc1Br)[C@@H]1CC(=O)NC1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50269943
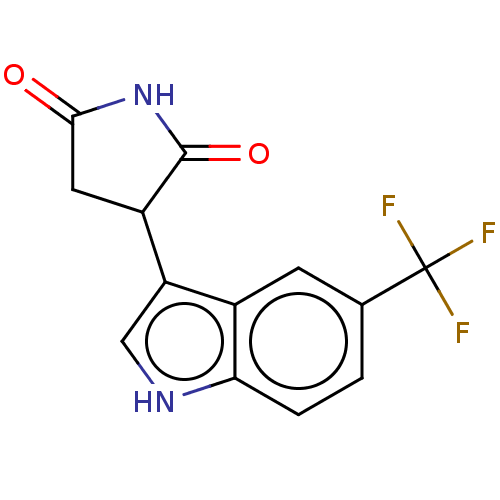
(CHEMBL4073619)Show InChI InChI=1S/C13H9F3N2O2/c14-13(15,16)6-1-2-10-7(3-6)9(5-17-10)8-4-11(19)18-12(8)20/h1-3,5,8,17H,4H2,(H,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM312064

((-)-(R)-3-(1H-indol-3- yl)pyrrolidine-2,5-dione | ...) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM312064

((-)-(R)-3-(1H-indol-3- yl)pyrrolidine-2,5-dione | ...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM312064

((-)-(R)-3-(1H-indol-3- yl)pyrrolidine-2,5-dione | ...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM312064

((-)-(R)-3-(1H-indol-3- yl)pyrrolidine-2,5-dione | ...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM312064

((-)-(R)-3-(1H-indol-3- yl)pyrrolidine-2,5-dione | ...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM310197

(US10945994, Compound 2a | US9603836, Compound 2a |...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of IDO-1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine production after 16 to 24 hrs by PDMAB method |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
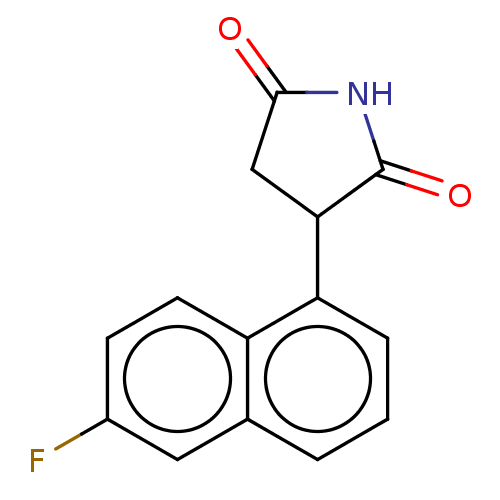
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM312087

(3-(naphthalen-1-yl)- pyrrolidine-2,5-dione | US960...)Show InChI InChI=1S/C14H11NO2/c16-13-8-12(14(17)15-13)11-7-3-5-9-4-1-2-6-10(9)11/h1-7,12H,8H2,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM310197

(US10945994, Compound 2a | US9603836, Compound 2a |...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using tryptophan as substrate after 22 mins by LC-MS/MS method |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM312081
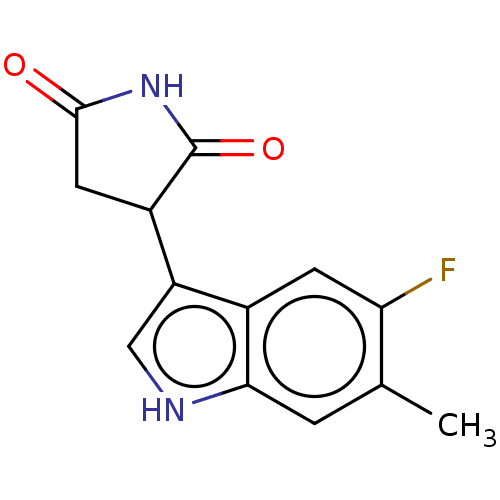
(3-(5-fluoro-6-methyl- 1H-indol-3-yl)- pyrrolidine-...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM312071
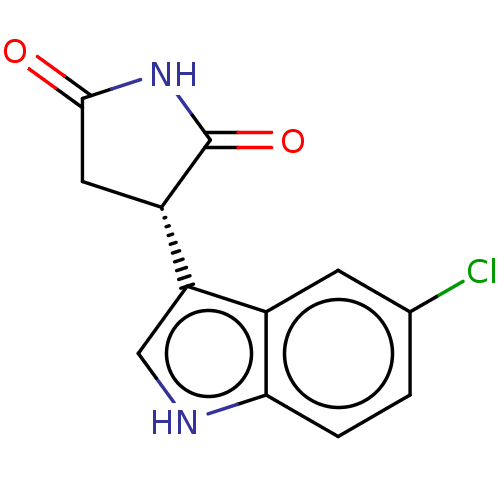
(US9603836, Compound 6a | US9949951, 6a) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50269915

(CHEMBL4080434)Show InChI InChI=1S/C14H10ClNO2/c15-9-4-5-10-8(6-9)2-1-3-11(10)12-7-13(17)16-14(12)18/h1-6,12H,7H2,(H,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50269916
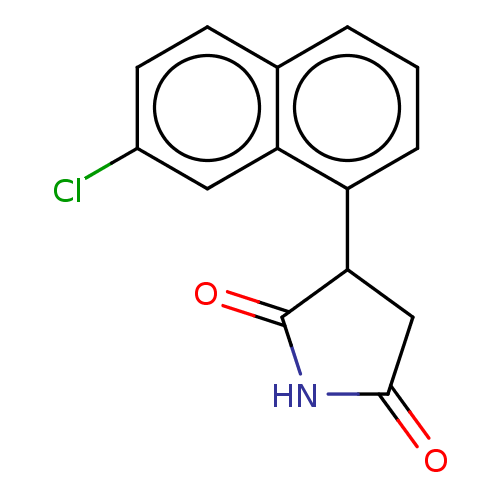
(CHEMBL4098167)Show InChI InChI=1S/C14H10ClNO2/c15-9-5-4-8-2-1-3-10(11(8)6-9)12-7-13(17)16-14(12)18/h1-6,12H,7H2,(H,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50269917
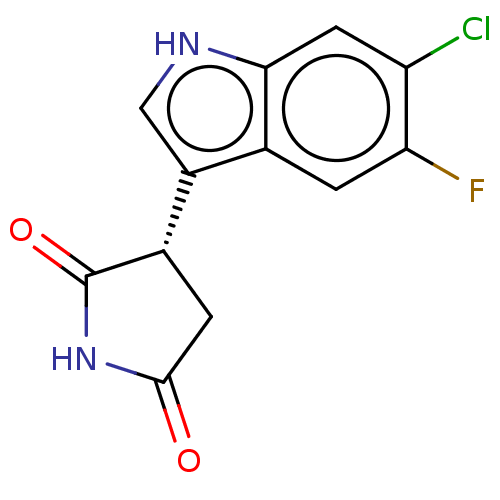
(CHEMBL4075749)Show SMILES Fc1cc2c(c[nH]c2cc1Cl)[C@@H]1CC(=O)NC1=O |r| Show InChI InChI=1S/C12H8ClFN2O2/c13-8-3-10-5(1-9(8)14)7(4-15-10)6-2-11(17)16-12(6)18/h1,3-4,6,15H,2H2,(H,16,17,18)/t6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50269931

(CHEMBL4099318)Show InChI InChI=1S/C13H12N2O3/c1-18-7-2-3-8-10(6-14-11(8)4-7)9-5-12(16)15-13(9)17/h2-4,6,9,14H,5H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM312077
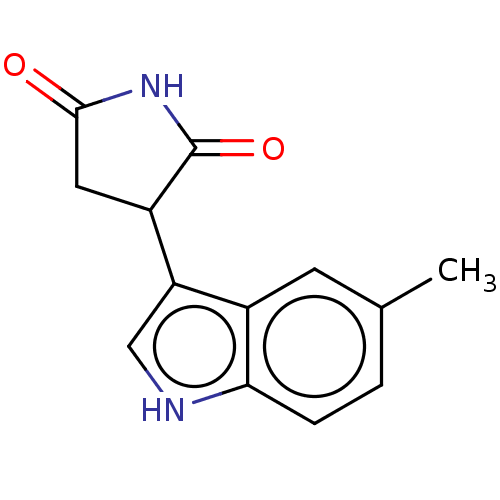
(3-(5-methyl-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50269920
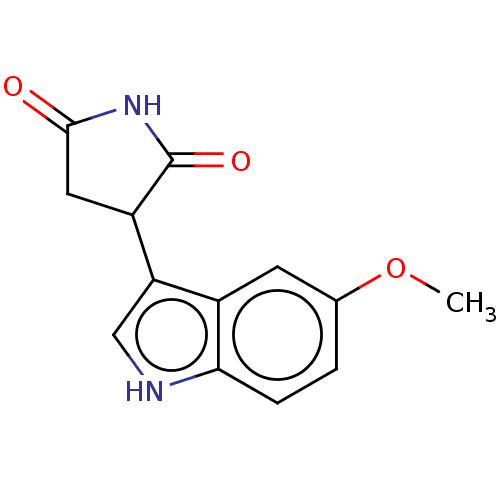
(CHEMBL4065171)Show InChI InChI=1S/C13H12N2O3/c1-18-7-2-3-11-8(4-7)10(6-14-11)9-5-12(16)15-13(9)17/h2-4,6,9,14H,5H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM310197

(US10945994, Compound 2a | US9603836, Compound 2a |...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM312085
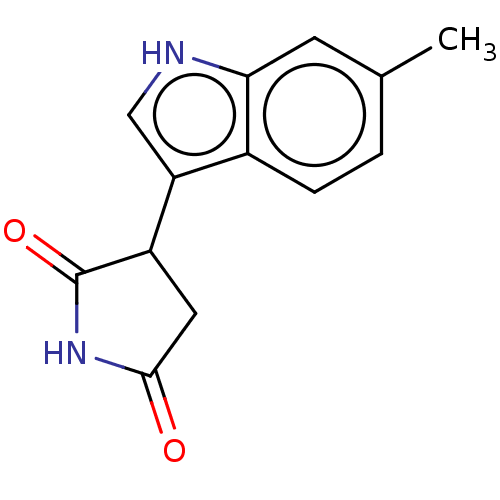
(3-(6-methyl-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM309529

(3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 6.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of BCRP (unknown origin) transfected in MDCK cells assessed as decrease in pitavastatin transport |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM309529

(3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM312065
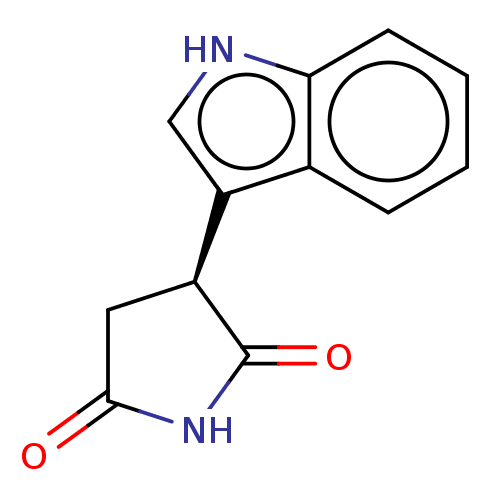
(US9603836, Compound 4a | US9949951, 4a) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO-1 using L-Trp as substrate after 15 mins by PDMAB-based assay |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM309529

(3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM309529

(3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM309529

(3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM309529

(3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM309529

(3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human TDO2 using tryptophan as substrate after 22 mins by LC-MS/MS method |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM310195

((-)-(R)-3-(5-fluoro-1H- indol-3-yl)pyrrolidine- 2,...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity to ferric form of human recombinant IDO-1 in absence of oxygen |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM310195

((-)-(R)-3-(5-fluoro-1H- indol-3-yl)pyrrolidine- 2,...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity to ferric form of human recombinant IDO-1 in absence of oxygen and presence of tryptophan |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM309529

(3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.23E+4 | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity to ferric form of human recombinant IDO-1 |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM309529

(3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity to ferrous form of human recombinant IDO-1 |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM309529

(3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity to ferric form of human recombinant IDO-1 in absence of oxygen |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM310195

((-)-(R)-3-(5-fluoro-1H- indol-3-yl)pyrrolidine- 2,...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity to ferrous form of human recombinant IDO-1 |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM310195

((-)-(R)-3-(5-fluoro-1H- indol-3-yl)pyrrolidine- 2,...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a |
iTeos Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity to ferric form of human recombinant IDO-1 |
J Med Chem 60: 9617-9629 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00974
BindingDB Entry DOI: 10.7270/Q2ZP48M3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data