 Found 18 hits of Enzyme Inhibition Constant Data
Found 18 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Albumin
(Homo sapiens (Human)) | BDBM50517250
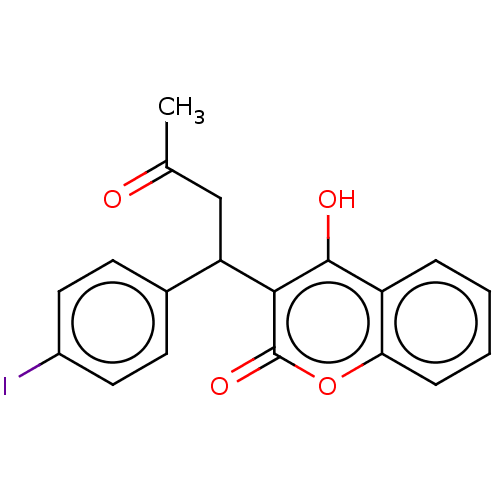
(CHEMBL4554026)Show InChI InChI=1S/C19H15IO4/c1-11(21)10-15(12-6-8-13(20)9-7-12)17-18(22)14-4-2-3-5-16(14)24-19(17)23/h2-9,15,22H,10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lavis
Curated by ChEMBL
| Assay Description
Binding affinity to human serum albumin assessed as inhibition constant by NMR spectroscopy |
J Med Chem 62: 2218-2244 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01210
BindingDB Entry DOI: 10.7270/Q2BP065B |
More data for this
Ligand-Target Pair | |
Albumin
(Homo sapiens (Human)) | BDBM50517250

(CHEMBL4554026)Show InChI InChI=1S/C19H15IO4/c1-11(21)10-15(12-6-8-13(20)9-7-12)17-18(22)14-4-2-3-5-16(14)24-19(17)23/h2-9,15,22H,10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lavis
Curated by ChEMBL
| Assay Description
Binding affinity to human serum albumin by fluorescence-based assay |
J Med Chem 62: 2218-2244 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01210
BindingDB Entry DOI: 10.7270/Q2BP065B |
More data for this
Ligand-Target Pair | |
Complement factor D
(Homo sapiens (Human)) | BDBM50236213
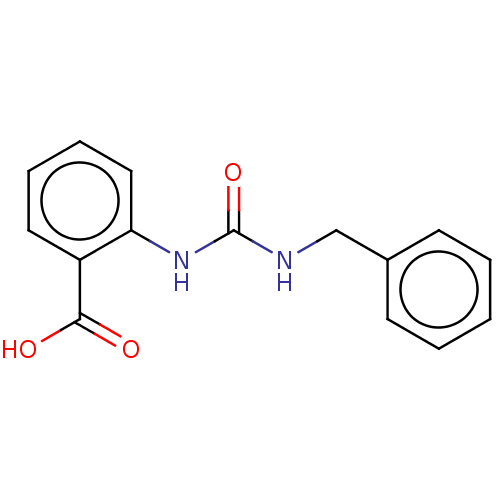
(2-(3-Benzylureido)Benzoic Acid | CHEMBL561499)Show InChI InChI=1S/C15H14N2O3/c18-14(19)12-8-4-5-9-13(12)17-15(20)16-10-11-6-2-1-3-7-11/h1-9H,10H2,(H,18,19)(H2,16,17,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lavis
Curated by ChEMBL
| Assay Description
Binding affinity to factor D (unknown origin) by 19F-NMR spectroscopy |
J Med Chem 62: 2218-2244 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01210
BindingDB Entry DOI: 10.7270/Q2BP065B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Complement factor D
(Homo sapiens (Human)) | BDBM50236214
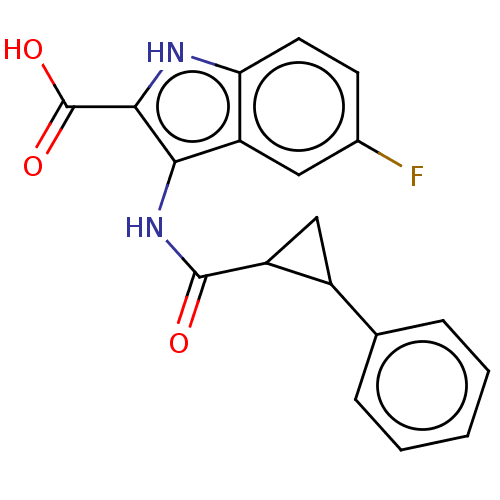
(CHEMBL4089211)Show SMILES OC(=O)c1[nH]c2ccc(F)cc2c1NC(=O)C1CC1c1ccccc1 Show InChI InChI=1S/C19H15FN2O3/c20-11-6-7-15-14(8-11)16(17(21-15)19(24)25)22-18(23)13-9-12(13)10-4-2-1-3-5-10/h1-8,12-13,21H,9H2,(H,22,23)(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lavis
Curated by ChEMBL
| Assay Description
Binding affinity to factor D (unknown origin) by 19F-NMR spectroscopy |
J Med Chem 62: 2218-2244 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01210
BindingDB Entry DOI: 10.7270/Q2BP065B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphopantetheine adenylyltransferase
(Escherichia coli) | BDBM50517256
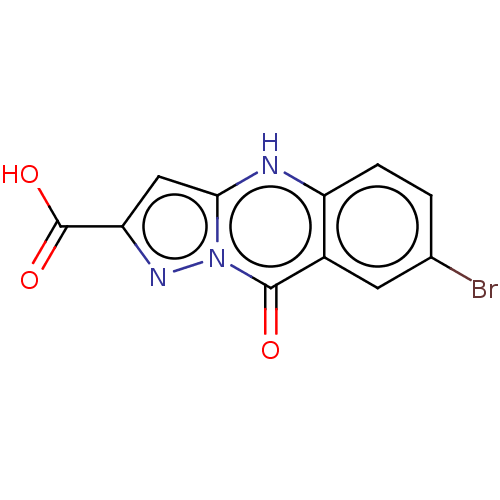
(CHEMBL4446301)Show InChI InChI=1S/C11H6BrN3O3/c12-5-1-2-7-6(3-5)10(16)15-9(13-7)4-8(14-15)11(17)18/h1-4,13H,(H,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lavis
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli PPAT after 5 mins by reverse ATP-generating-luciferase assay |
J Med Chem 62: 2218-2244 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01210
BindingDB Entry DOI: 10.7270/Q2BP065B |
More data for this
Ligand-Target Pair | |
Complement factor D
(Homo sapiens (Human)) | BDBM203865

(Methyl (S)-2-((2-((3-(trifluoromethoxy)phenyl)carb...)Show SMILES COC(=O)c1ccccc1CNC(=O)N1CCC[C@H]1C(=O)Nc1cccc(OC(F)(F)F)c1 Show InChI InChI=1S/C22H22F3N3O5/c1-32-20(30)17-9-3-2-6-14(17)13-26-21(31)28-11-5-10-18(28)19(29)27-15-7-4-8-16(12-15)33-22(23,24)25/h2-4,6-9,12,18H,5,10-11,13H2,1H3,(H,26,31)(H,27,29)/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lavis
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human factor D expressed in Escherichia coli using Z-Lys-thiobenzyl as substrate after 1 hr by spectrofluorimetry |
J Med Chem 62: 2218-2244 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01210
BindingDB Entry DOI: 10.7270/Q2BP065B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphopantetheine adenylyltransferase
(Escherichia coli) | BDBM50517257
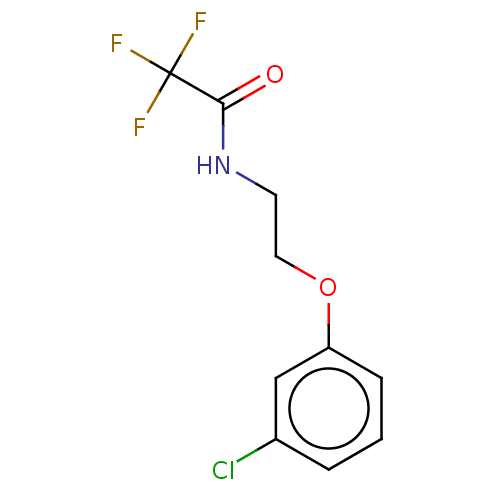
(CHEMBL4584904)Show InChI InChI=1S/C10H9ClF3NO2/c11-7-2-1-3-8(6-7)17-5-4-15-9(16)10(12,13)14/h1-3,6H,4-5H2,(H,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lavis
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli PPAT after 5 mins by reverse ATP-generating-luciferase assay |
J Med Chem 62: 2218-2244 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01210
BindingDB Entry DOI: 10.7270/Q2BP065B |
More data for this
Ligand-Target Pair | |
Phosphopantetheine adenylyltransferase
(Escherichia coli) | BDBM50517254
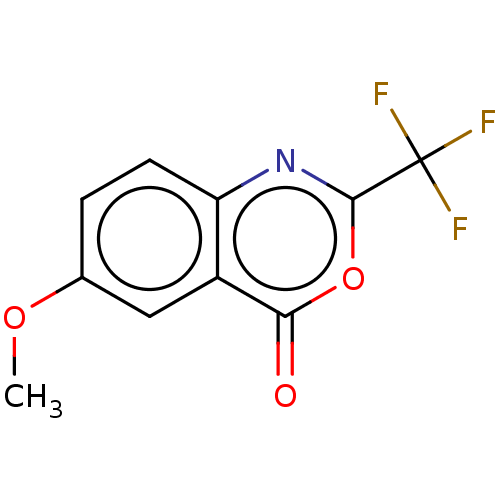
(CHEMBL4553617)Show InChI InChI=1S/C10H6F3NO3/c1-16-5-2-3-7-6(4-5)8(15)17-9(14-7)10(11,12)13/h2-4H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lavis
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli PPAT after 5 mins by reverse ATP-generating-luciferase assay |
J Med Chem 62: 2218-2244 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01210
BindingDB Entry DOI: 10.7270/Q2BP065B |
More data for this
Ligand-Target Pair | |
Albumin
(Homo sapiens (Human)) | BDBM50517249
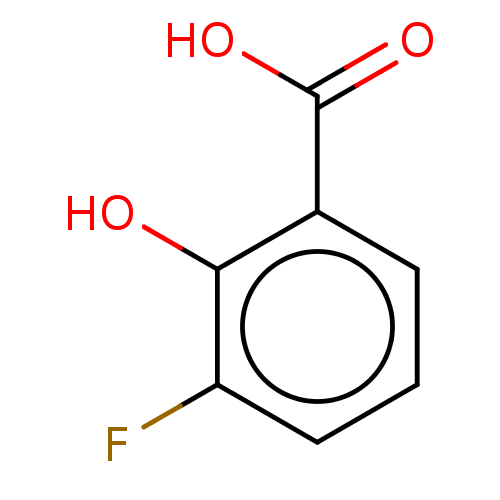
(CHEMBL3244575)Show InChI InChI=1S/C7H5FO3/c8-5-3-1-2-4(6(5)9)7(10)11/h1-3,9H,(H,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.18E+4 | n/a | n/a | n/a | n/a | n/a |
Lavis
Curated by ChEMBL
| Assay Description
Binding affinity to human serum albumin by NMR spectroscopy |
J Med Chem 62: 2218-2244 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01210
BindingDB Entry DOI: 10.7270/Q2BP065B |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50131433
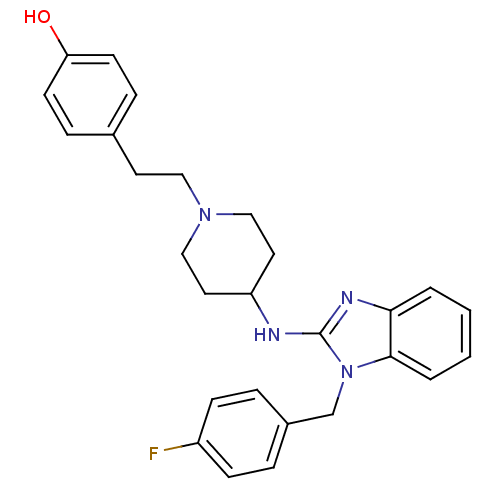
(4-(2-(4-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-...)Show SMILES Oc1ccc(CCN2CCC(CC2)Nc2nc3ccccc3n2Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C27H29FN4O/c28-22-9-5-21(6-10-22)19-32-26-4-2-1-3-25(26)30-27(32)29-23-14-17-31(18-15-23)16-13-20-7-11-24(33)12-8-20/h1-12,23,33H,13-19H2,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a |
Lavis
Curated by ChEMBL
| Assay Description
Binding affinity to Nurr1 (unknown origin) by 1H-STD-NMR spectroscopy |
J Med Chem 62: 2218-2244 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01210
BindingDB Entry DOI: 10.7270/Q2BP065B |
More data for this
Ligand-Target Pair | |
CD209 antigen
(Homo sapiens (Human)) | BDBM50517251

(CHEMBL1503347)Show SMILES COc1ccccc1N1CCN(CC1)C(=O)c1ccc2NC(CSCc3ccccc3C)C(=O)Nc2c1 Show InChI InChI=1S/C29H32N4O3S/c1-20-7-3-4-8-22(20)18-37-19-25-28(34)31-24-17-21(11-12-23(24)30-25)29(35)33-15-13-32(14-16-33)26-9-5-6-10-27(26)36-2/h3-12,17,25,30H,13-16,18-19H2,1-2H3,(H,31,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a |
Lavis
Curated by ChEMBL
| Assay Description
Binding affinity to human DC-SIGN ECD expressed in Escherichia coli BL21(DE3) after 1 hr by Man-Fl-BSA assay |
J Med Chem 62: 2218-2244 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01210
BindingDB Entry DOI: 10.7270/Q2BP065B |
More data for this
Ligand-Target Pair | |
Bifunctional UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase
(Homo sapiens) | BDBM50517252
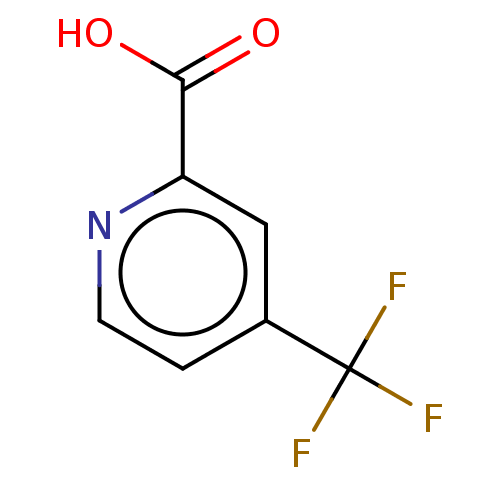
(CHEMBL4457121)Show InChI InChI=1S/C7H4F3NO2/c8-7(9,10)4-1-2-11-5(3-4)6(12)13/h1-3H,(H,12,13) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 9.00E+5 | n/a | n/a | n/a | n/a | n/a |
Lavis
Curated by ChEMBL
| Assay Description
Binding affinity to His-tagged human MNK expressed in Escherichia coli BL21(DE3) in presence of ATP by 9F-NMR spectroscopy analysis |
J Med Chem 62: 2218-2244 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01210
BindingDB Entry DOI: 10.7270/Q2BP065B |
More data for this
Ligand-Target Pair | |
CD209 antigen
(Homo sapiens (Human)) | BDBM108233
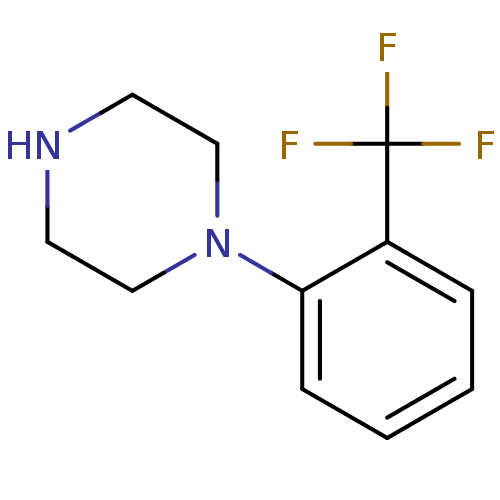
(1‐[2‐(trifluoromethyl)phenyl]piperazin...)Show InChI InChI=1S/C11H13F3N2/c12-11(13,14)9-3-1-2-4-10(9)16-7-5-15-6-8-16/h1-4,15H,5-8H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.60E+6 | n/a | n/a | n/a | n/a | n/a |
Lavis
Curated by ChEMBL
| Assay Description
Binding affinity to human DC-SIGN ECD expressed in Escherichia coli BL21(DE3) after 1 hr by Man-Fl-BSA assay |
J Med Chem 62: 2218-2244 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01210
BindingDB Entry DOI: 10.7270/Q2BP065B |
More data for this
Ligand-Target Pair | |
CD209 antigen
(Homo sapiens (Human)) | BDBM50517255

(CHEMBL4442395)Show InChI InChI=1S/C8H6FNO2/c9-5-1-2-6-7(3-5)12-4-8(11)10-6/h1-3H,4H2,(H,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.10E+6 | n/a | n/a | n/a | n/a | n/a |
Lavis
Curated by ChEMBL
| Assay Description
Binding affinity to human DC-SIGN ECD expressed in Escherichia coli BL21(DE3) after 1 hr by Man-Fl-BSA assay |
J Med Chem 62: 2218-2244 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01210
BindingDB Entry DOI: 10.7270/Q2BP065B |
More data for this
Ligand-Target Pair | |
Bifunctional UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase
(Homo sapiens) | BDBM50517253
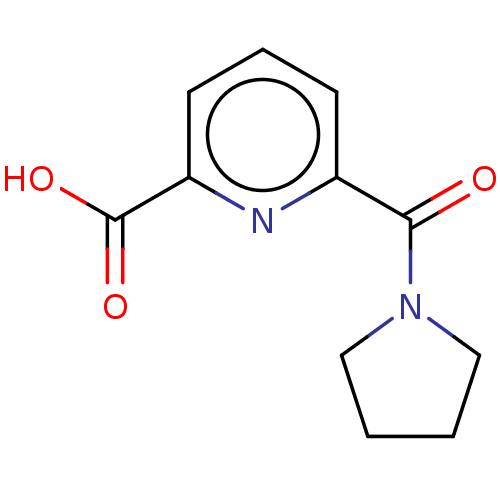
(CHEMBL4435406)Show InChI InChI=1S/C11H12N2O3/c14-10(13-6-1-2-7-13)8-4-3-5-9(12-8)11(15)16/h3-5H,1-2,6-7H2,(H,15,16) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a |
Lavis
Curated by ChEMBL
| Assay Description
Binding affinity to His-tagged human MNK expressed in Escherichia coli BL21(DE3) in presence of ATP by 9F-NMR spectroscopy analysis |
J Med Chem 62: 2218-2244 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01210
BindingDB Entry DOI: 10.7270/Q2BP065B |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50117925
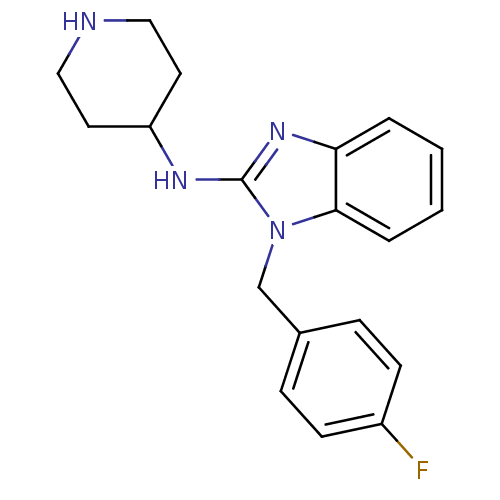
(1-(4-fluorobenzyl)-N-(piperidin-4-yl)-1H-benzo[d]i...)Show InChI InChI=1S/C19H21FN4/c20-15-7-5-14(6-8-15)13-24-18-4-2-1-3-17(18)23-19(24)22-16-9-11-21-12-10-16/h1-8,16,21H,9-13H2,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a |
Lavis
Curated by ChEMBL
| Assay Description
Binding affinity to Nurr1 (unknown origin) by 1H-STD-NMR spectroscopy |
J Med Chem 62: 2218-2244 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01210
BindingDB Entry DOI: 10.7270/Q2BP065B |
More data for this
Ligand-Target Pair | |
Albumin
(Homo sapiens (Human)) | BDBM50517249

(CHEMBL3244575)Show InChI InChI=1S/C7H5FO3/c8-5-3-1-2-4(6(5)9)7(10)11/h1-3,9H,(H,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a |
Lavis
Curated by ChEMBL
| Assay Description
Binding affinity to human serum albumin by ITC |
J Med Chem 62: 2218-2244 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01210
BindingDB Entry DOI: 10.7270/Q2BP065B |
More data for this
Ligand-Target Pair | |
CD209 antigen
(Homo sapiens (Human)) | BDBM50004316

((4-Fluoro-phenyl)-piperidin-4-yl-methanone | (4-fl...)Show InChI InChI=1S/C12H14FNO/c13-11-3-1-9(2-4-11)12(15)10-5-7-14-8-6-10/h1-4,10,14H,5-8H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.20E+6 | n/a | n/a | n/a | n/a | n/a |
Lavis
Curated by ChEMBL
| Assay Description
Binding affinity to human DC-SIGN ECD expressed in Escherichia coli BL21(DE3) after 1 hr by Man-Fl-BSA assay |
J Med Chem 62: 2218-2244 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01210
BindingDB Entry DOI: 10.7270/Q2BP065B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data