

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor A (RAT) | BDBM50049189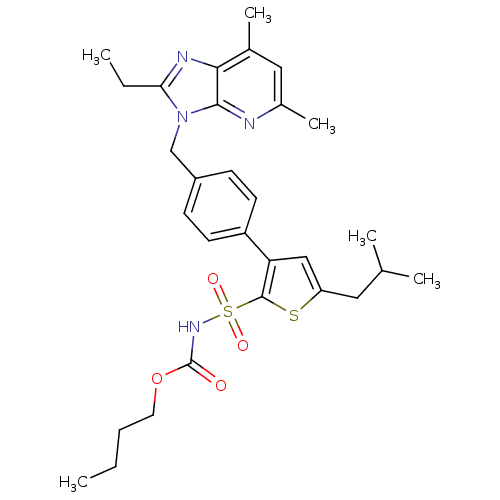 (3-[4-(2-Ethyl-57-dimethyl-imidazo[45-b]pyridin-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL) Curated by ChEMBL | Assay Description Displacement of [125I]Ang II from AT1 receptor in rat liver membranes | J Med Chem 63: 1978-1995 (2020) Article DOI: 10.1021/acs.jmedchem.9b01780 BindingDB Entry DOI: 10.7270/Q20868NG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50282396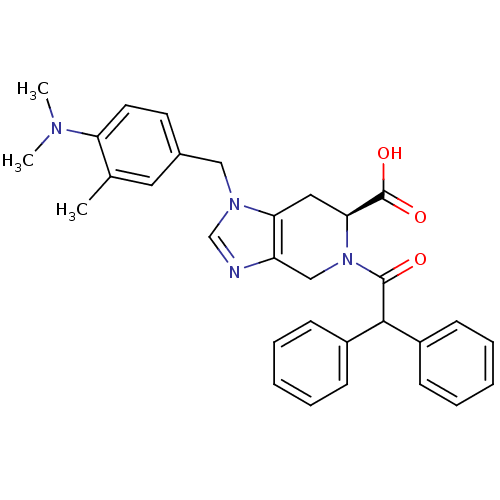 ((S)-1-(4-Dimethylamino-3-methyl-benzyl)-5-diphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL) Curated by ChEMBL | Assay Description Inhibition of human AT1 receptor | J Med Chem 63: 1978-1995 (2020) Article DOI: 10.1021/acs.jmedchem.9b01780 BindingDB Entry DOI: 10.7270/Q20868NG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50514581 (CHEMBL1885579) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL) Curated by ChEMBL | Assay Description Displacement of [125I]Ang II from AT1 receptor in rat SMC membranes incubated for 60 mins by gamma counting method | J Med Chem 63: 1978-1995 (2020) Article DOI: 10.1021/acs.jmedchem.9b01780 BindingDB Entry DOI: 10.7270/Q20868NG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50156173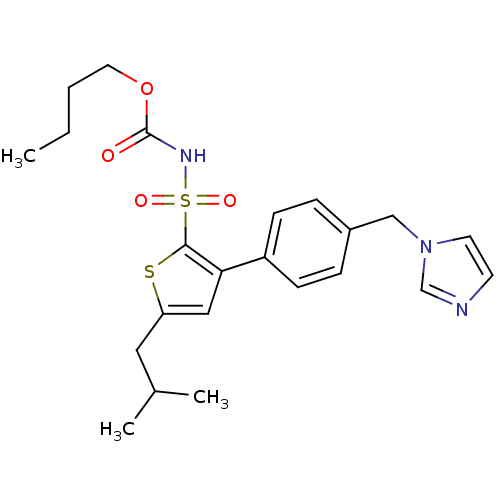 ((1-((2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL) Curated by ChEMBL | Assay Description Displacement of [125I]Ang II from AT1 receptor in rat liver membranes | J Med Chem 63: 1978-1995 (2020) Article DOI: 10.1021/acs.jmedchem.9b01780 BindingDB Entry DOI: 10.7270/Q20868NG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50282396 ((S)-1-(4-Dimethylamino-3-methyl-benzyl)-5-diphenyl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL) Curated by ChEMBL | Assay Description Inhibition of human AT2 receptor | J Med Chem 63: 1978-1995 (2020) Article DOI: 10.1021/acs.jmedchem.9b01780 BindingDB Entry DOI: 10.7270/Q20868NG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene Mas (Homo sapiens) | BDBM50514583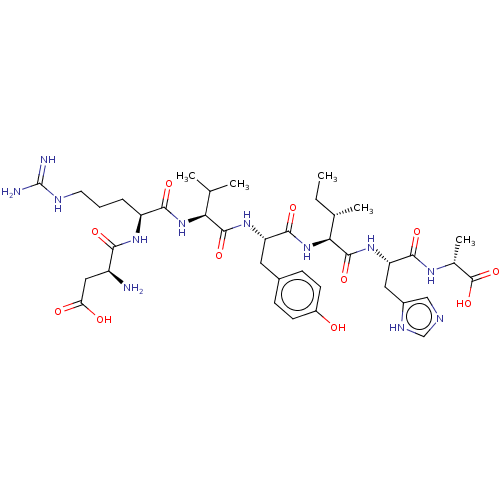 (CHEMBL4578721) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL) Curated by ChEMBL | Assay Description Antagonist activity at N-terminal c-Myc tagged human MasR expressed in HEK293T cells by cAMP accumulation based assay | J Med Chem 63: 1978-1995 (2020) Article DOI: 10.1021/acs.jmedchem.9b01780 BindingDB Entry DOI: 10.7270/Q20868NG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21489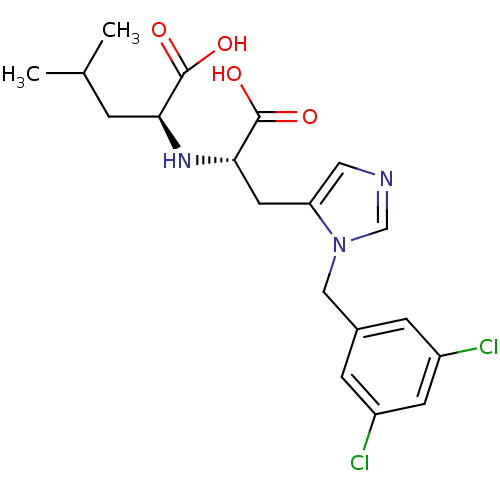 ((2S)-2-{[(1S)-1-carboxy-2-{1-[(3,5-dichlorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL) Curated by ChEMBL | Assay Description Inhibition of ACE2 (unknown origin) | J Med Chem 63: 1978-1995 (2020) Article DOI: 10.1021/acs.jmedchem.9b01780 BindingDB Entry DOI: 10.7270/Q20868NG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50369183 (CHEMBL290214 | L-163491) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL) Curated by ChEMBL | Assay Description Displacement of [125I]Ang II from AT1 receptor in rat liver membranes | J Med Chem 63: 1978-1995 (2020) Article DOI: 10.1021/acs.jmedchem.9b01780 BindingDB Entry DOI: 10.7270/Q20868NG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene Mas (Homo sapiens) | BDBM50514584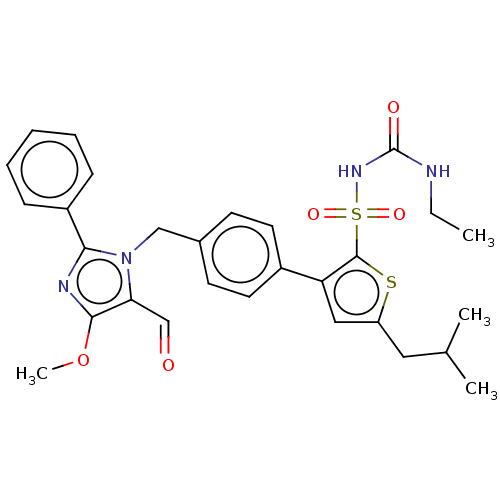 (CHEMBL4303593) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL) Curated by ChEMBL | Assay Description Agonist activity at N-terminal c-Myc tagged human MasR expressed in HEK293T cells assessed as reduction in forskolin-induced cAMP accumulation | J Med Chem 63: 1978-1995 (2020) Article DOI: 10.1021/acs.jmedchem.9b01780 BindingDB Entry DOI: 10.7270/Q20868NG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50049182 ((S)-5-Benzyloxy-2-diphenylacetyl-6-methoxy-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL) Curated by ChEMBL | Assay Description Inhibition of human AT1 receptor | J Med Chem 63: 1978-1995 (2020) Article DOI: 10.1021/acs.jmedchem.9b01780 BindingDB Entry DOI: 10.7270/Q20868NG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50514582 (CHEMBL155836) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL) Curated by ChEMBL | Assay Description Inhibition of rat AT2 receptor | J Med Chem 63: 1978-1995 (2020) Article DOI: 10.1021/acs.jmedchem.9b01780 BindingDB Entry DOI: 10.7270/Q20868NG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50514585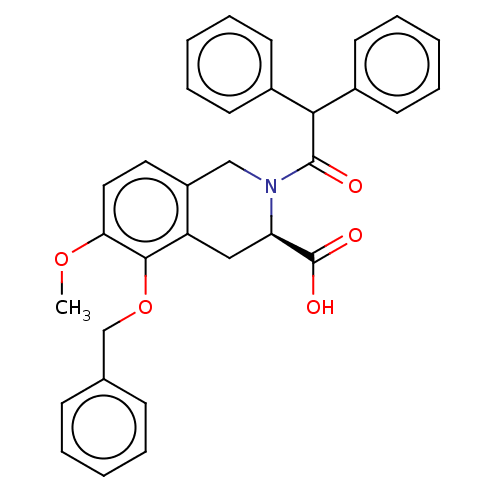 (CHEMBL4448705) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL) Curated by ChEMBL | Assay Description Inhibition of human AT1 receptor | J Med Chem 63: 1978-1995 (2020) Article DOI: 10.1021/acs.jmedchem.9b01780 BindingDB Entry DOI: 10.7270/Q20868NG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50049182 ((S)-5-Benzyloxy-2-diphenylacetyl-6-methoxy-1,2,3,4...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL) Curated by ChEMBL | Assay Description Inhibition of human AT2 receptor | J Med Chem 63: 1978-1995 (2020) Article DOI: 10.1021/acs.jmedchem.9b01780 BindingDB Entry DOI: 10.7270/Q20868NG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50514582 (CHEMBL155836) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL) Curated by ChEMBL | Assay Description Inhibition of rat AT1 receptor | J Med Chem 63: 1978-1995 (2020) Article DOI: 10.1021/acs.jmedchem.9b01780 BindingDB Entry DOI: 10.7270/Q20868NG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||