

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM201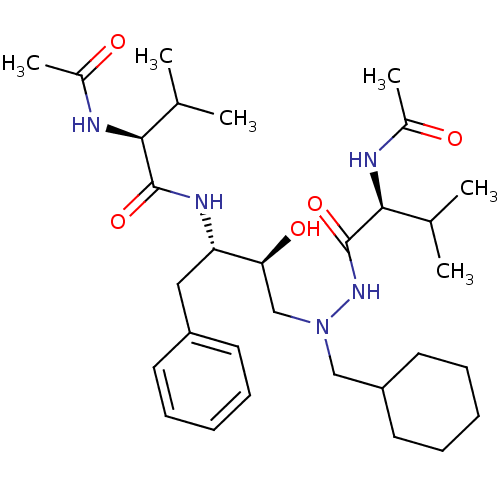 ((2S)-N-[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease at pH 6 using 164 microM peptide substrate | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281324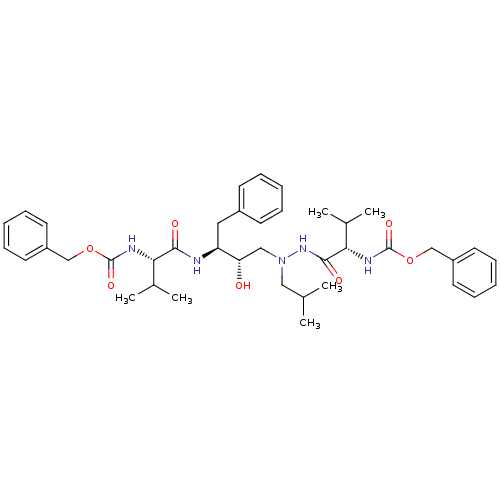 (((S)-1-{N'-[(2S,3S)-3-((S)-2-Benzyloxycarbonylamin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease at pH 6 using 164 microM peptide substrate | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281333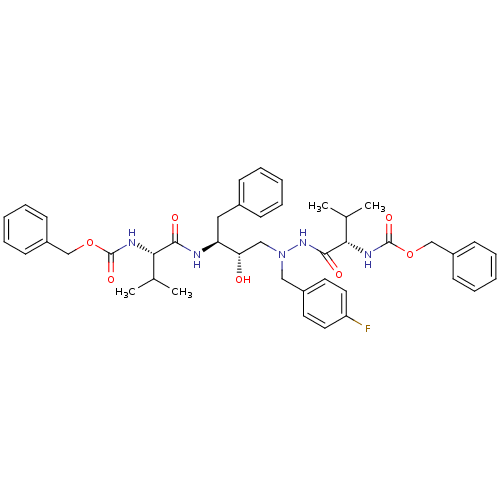 (CHEMBL97512 | {(S)-1-[N'-[(2S,3S)-3-((S)-2-Benzylo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease at pH 6 using 164 microM peptide substrate | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281332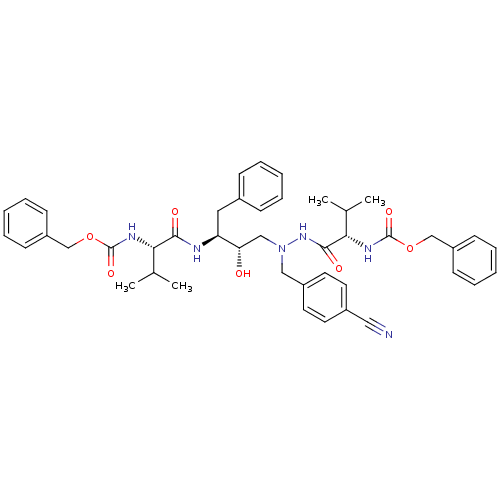 (CHEMBL95462 | {(S)-1-[N'-[(2S,3S)-3-((S)-2-Benzylo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease at pH 6 using 164 microM peptide substrate | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281328 ((S)-2-Acetylamino-N-{(1S,2S)-3-[N'-((S)-2-acetylam...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease at pH 6 using 164 microM peptide substrate | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281330 ((S)-2-Acetylamino-N-{(1S,2S)-3-[N'-((R)-2-acetylam...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease at pH 6 using 164 microM peptide substrate | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281335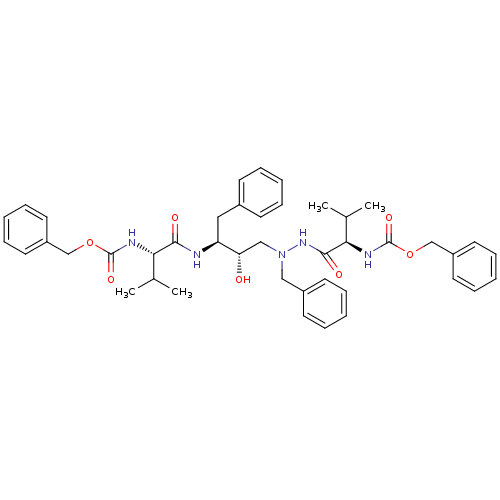 (((R)-1-{N'-Benzyl-N'-[(2S,3S)-3-((S)-2-benzyloxyca...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 33 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease at pH 6 using 164 microM peptide substrate | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281327 ((S)-2-Acetylamino-N-{(1S,2S)-3-[N'-((S)-2-acetylam...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease at pH 6 using 164 microM peptide substrate | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281337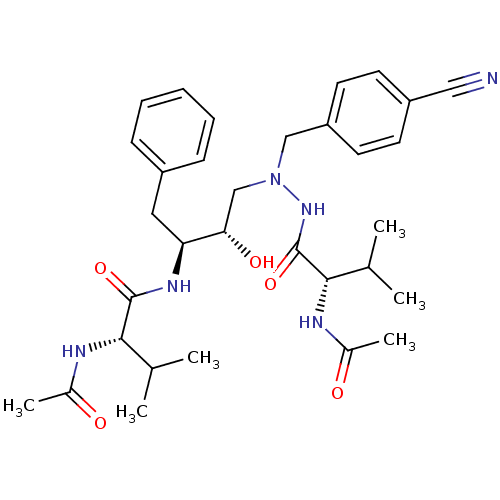 ((S)-2-Acetylamino-N-{(1S,2S)-3-[N'-((S)-2-acetylam...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease at pH 6 using 164 microM peptide substrate | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281329 (CHEMBL97146 | quinoline-2-carboxylic acid {(S)-1-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease at pH 6 using 164 microM peptide substrate | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281323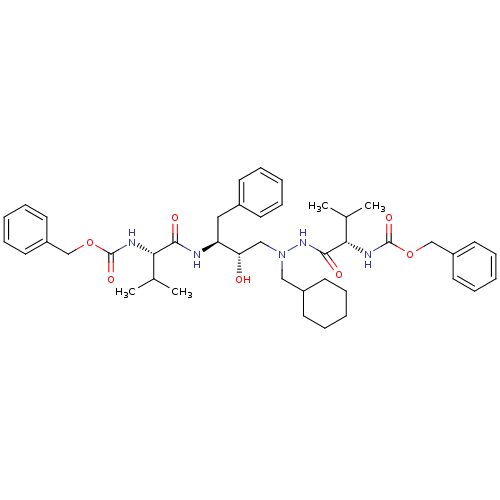 (((S)-1-{N'-[(2S,3S)-3-((S)-2-Benzyloxycarbonylamin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 95 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease at pH 6 using 164 microM peptide substrate | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281334 ((S)-N-{(1S,2S)-1-Benzyl-3-[N-benzyl-N'-((R)-3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 360 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease at pH 6 using 164 microM peptide substrate | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281338 ((S)-N-((1S,2S)-1-Benzyl-3-{N-benzyl-N'-[(R)-3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 500 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease at pH 6 using 164 microM peptide substrate | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM201 ((2S)-N-[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its inhibitory activity against human pepsin | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastricsin (Homo sapiens (Human)) | BDBM201 ((2S)-N-[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its inhibitory activity against human gastricsin | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM201 ((2S)-N-[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-ac...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its inhibitory activity against human cathepsin E | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281336 (CHEMBL327985 | morpholine-4-carboxylic acid {(S)-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease at pH 6 using 164 microM peptide substrate | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM201 ((2S)-N-[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-ac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its inhibitory activity against human cathepsin D | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281331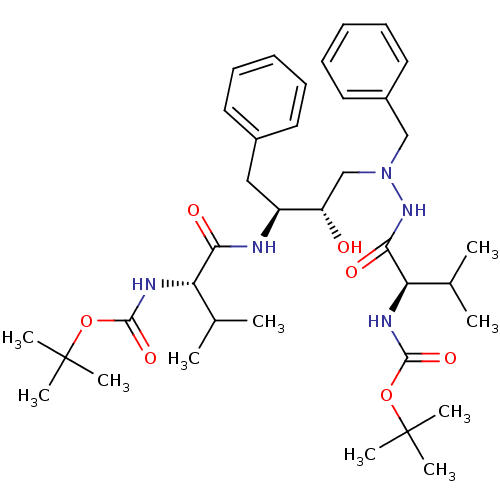 (((R)-1-{N'-Benzyl-N'-[(2S,3S)-3-((S)-2-tert-butoxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease at pH 6 using 164 microM peptide substrate | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM201 ((2S)-N-[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | >5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its inhibitory activity against human renin | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281326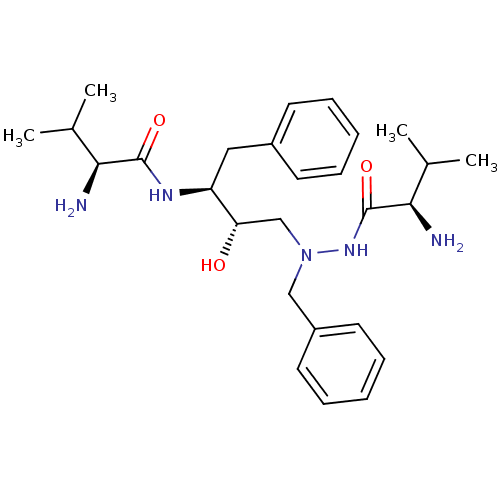 (2-Amino-N-{(S)-3-[N'-((R)-2-amino-3-methyl-butyryl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease at pH 6 using 164 microM peptide substrate | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50281325 (((S)-1-{N'-Benzyl-N'-[(2S,3S)-3-((R)-2-benzyloxyca...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease at pH 6 using 164 microM peptide substrate | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||