

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50286742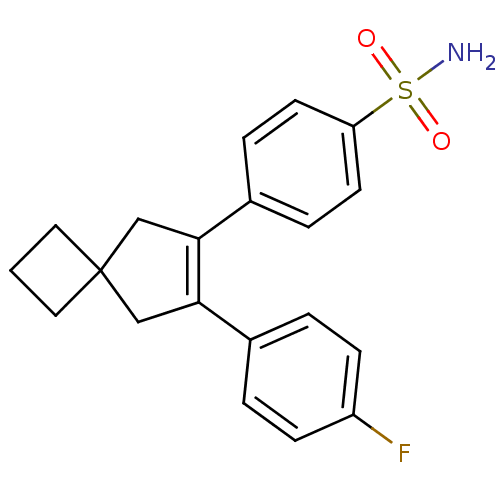 (4-[7-(4-Fluoro-phenyl)-spiro[3.4]oct-6-en-6-yl]-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory activity against inducible form of human recombinant Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 5: 867-872 (1995) Article DOI: 10.1016/0960-894X(95)00131-C BindingDB Entry DOI: 10.7270/Q2P26Z3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50049024 (4-[6-(4-Fluoro-phenyl)-spiro[2.4]hept-5-en-5-yl]-b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory activity against inducible form of human recombinant Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 5: 867-872 (1995) Article DOI: 10.1016/0960-894X(95)00131-C BindingDB Entry DOI: 10.7270/Q2P26Z3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50049022 (6-(4-Fluoro-phenyl)-7-(4-methanesulfonyl-phenyl)-s...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory activity against inducible form of human recombinant Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 5: 867-872 (1995) Article DOI: 10.1016/0960-894X(95)00131-C BindingDB Entry DOI: 10.7270/Q2P26Z3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50049041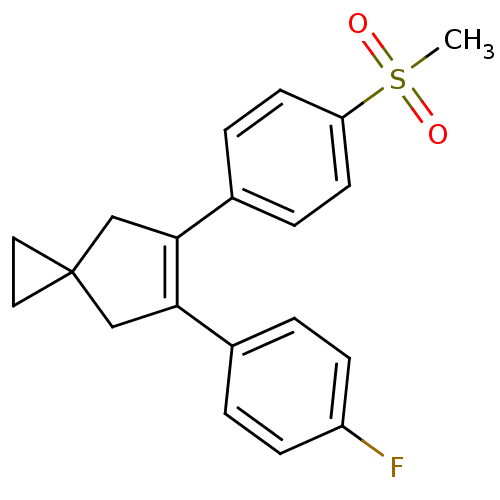 (5-(4-Fluoro-phenyl)-6-(4-methanesulfonyl-phenyl)-s...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory activity against inducible form of human recombinant Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 5: 867-872 (1995) Article DOI: 10.1016/0960-894X(95)00131-C BindingDB Entry DOI: 10.7270/Q2P26Z3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50037945 (1-[2-(4-fluorophenyl)-4,4-dimethyl-1-cyclopentenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory activity against inducible form of human recombinant Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 5: 867-872 (1995) Article DOI: 10.1016/0960-894X(95)00131-C BindingDB Entry DOI: 10.7270/Q2P26Z3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50029614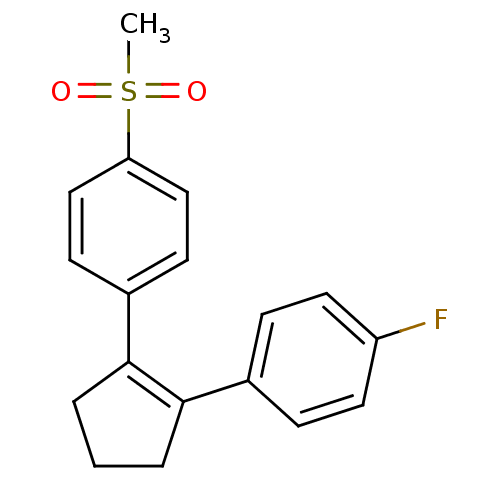 ((SC-57666)1-[2-(4-fluorophenyl)-1-cyclopentenyl]-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory activity against inducible form of human recombinant Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 5: 867-872 (1995) Article DOI: 10.1016/0960-894X(95)00131-C BindingDB Entry DOI: 10.7270/Q2P26Z3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50049039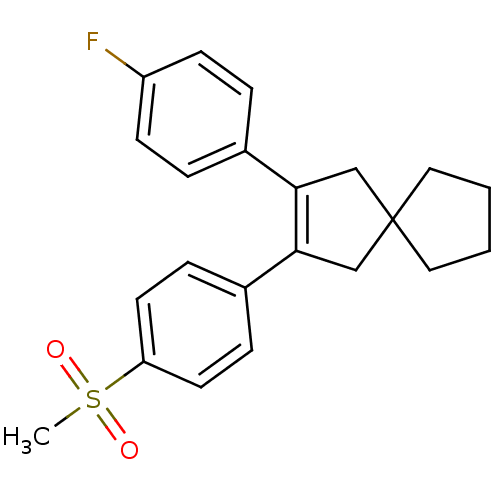 (2-(4-Fluoro-phenyl)-3-(4-methanesulfonyl-phenyl)-s...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory activity against inducible form of human recombinant Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 5: 867-872 (1995) Article DOI: 10.1016/0960-894X(95)00131-C BindingDB Entry DOI: 10.7270/Q2P26Z3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory activity against constitutive form of human recombinant Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 5: 867-872 (1995) Article DOI: 10.1016/0960-894X(95)00131-C BindingDB Entry DOI: 10.7270/Q2P26Z3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50029593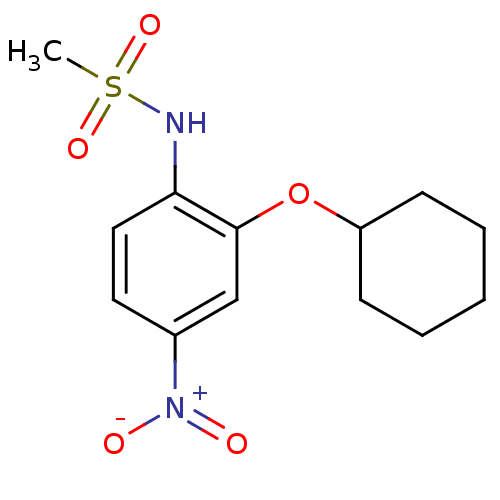 (CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory activity against inducible form of human recombinant cyclooxygenase (COX-2) | Bioorg Med Chem Lett 5: 867-872 (1995) Article DOI: 10.1016/0960-894X(95)00131-C BindingDB Entry DOI: 10.7270/Q2P26Z3Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50049024 (4-[6-(4-Fluoro-phenyl)-spiro[2.4]hept-5-en-5-yl]-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory activity against constitutive form of human recombinant Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 5: 867-872 (1995) Article DOI: 10.1016/0960-894X(95)00131-C BindingDB Entry DOI: 10.7270/Q2P26Z3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50286742 (4-[7-(4-Fluoro-phenyl)-spiro[3.4]oct-6-en-6-yl]-be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory activity against constitutive form of human recombinant Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 5: 867-872 (1995) Article DOI: 10.1016/0960-894X(95)00131-C BindingDB Entry DOI: 10.7270/Q2P26Z3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory activity against inducible form of human recombinant Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 5: 867-872 (1995) Article DOI: 10.1016/0960-894X(95)00131-C BindingDB Entry DOI: 10.7270/Q2P26Z3Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50049041 (5-(4-Fluoro-phenyl)-6-(4-methanesulfonyl-phenyl)-s...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory activity against constitutive form of human recombinant Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 5: 867-872 (1995) Article DOI: 10.1016/0960-894X(95)00131-C BindingDB Entry DOI: 10.7270/Q2P26Z3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50037945 (1-[2-(4-fluorophenyl)-4,4-dimethyl-1-cyclopentenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory activity against constitutive form of human recombinant Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 5: 867-872 (1995) Article DOI: 10.1016/0960-894X(95)00131-C BindingDB Entry DOI: 10.7270/Q2P26Z3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50049039 (2-(4-Fluoro-phenyl)-3-(4-methanesulfonyl-phenyl)-s...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory activity against constitutive form of human recombinant Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 5: 867-872 (1995) Article DOI: 10.1016/0960-894X(95)00131-C BindingDB Entry DOI: 10.7270/Q2P26Z3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50029593 (CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory activity against constitutive form of human recombinant cyclooxygenase (COX-1) | Bioorg Med Chem Lett 5: 867-872 (1995) Article DOI: 10.1016/0960-894X(95)00131-C BindingDB Entry DOI: 10.7270/Q2P26Z3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50049012 (2-(4-Fluoro-phenyl)-3-(4-methanesulfonyl-phenyl)-s...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory activity against constitutive form of human recombinant Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 5: 867-872 (1995) Article DOI: 10.1016/0960-894X(95)00131-C BindingDB Entry DOI: 10.7270/Q2P26Z3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50049022 (6-(4-Fluoro-phenyl)-7-(4-methanesulfonyl-phenyl)-s...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory activity against constitutive form of human recombinant Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 5: 867-872 (1995) Article DOI: 10.1016/0960-894X(95)00131-C BindingDB Entry DOI: 10.7270/Q2P26Z3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50049012 (2-(4-Fluoro-phenyl)-3-(4-methanesulfonyl-phenyl)-s...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory activity against inducible form of human recombinant Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 5: 867-872 (1995) Article DOI: 10.1016/0960-894X(95)00131-C BindingDB Entry DOI: 10.7270/Q2P26Z3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50029614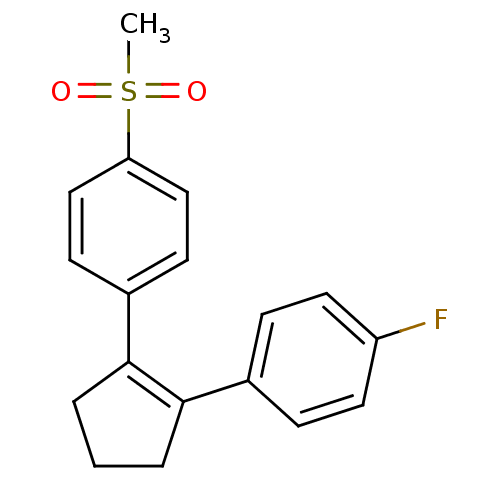 ((SC-57666)1-[2-(4-fluorophenyl)-1-cyclopentenyl]-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory activity against constitutive form of human recombinant Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 5: 867-872 (1995) Article DOI: 10.1016/0960-894X(95)00131-C BindingDB Entry DOI: 10.7270/Q2P26Z3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||