

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50017478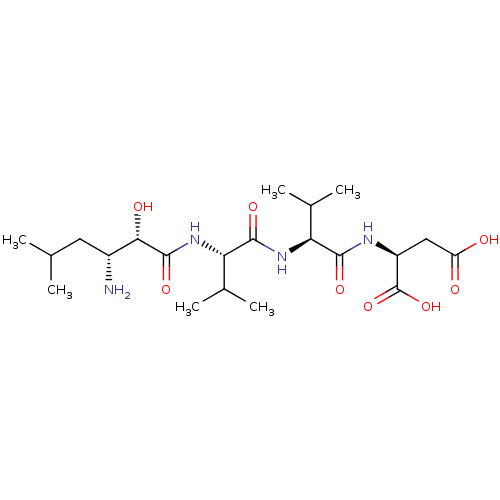 (Amastatin | CHEMBL28650 | Leu[1psi,CHOHCONH]ValVal...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of aminopeptidase M was determined and Ki* was reported which is obtained by the equatio... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50017478 (Amastatin | CHEMBL28650 | Leu[1psi,CHOHCONH]ValVal...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of Aminopeptidase M from porcine kidney was determined and Ki was reported which is obta... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50027041 (2-(3-Amino-2-hydroxy-5-methyl-hexanoylamino)-4-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Leucine aminopeptidase and the inhibition constant was determined after preincubating the enzyme and inh... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50027042 (3-Amino-2-hydroxy-5-methyl-hexanoic acid {2-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Aminopeptidase M (AP-M) and the inhibition constant was determined after preincubating the enzyme and in... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50017478 (Amastatin | CHEMBL28650 | Leu[1psi,CHOHCONH]ValVal...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | MMDB PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of Leucine aminopeptidase was determined and the Ki was reported which is = k2/k1 | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50017478 (Amastatin | CHEMBL28650 | Leu[1psi,CHOHCONH]ValVal...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | MMDB PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of aminopeptidase M was determined and Ki* was reported which is obtained by the equatio... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50027043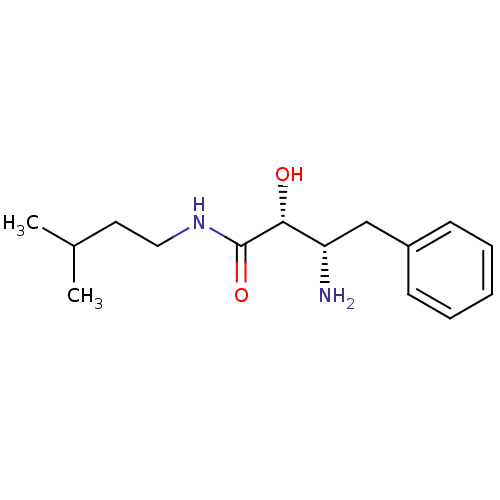 (3-Amino-2-hydroxy-N-(3-methyl-butyl)-4-phenyl-buty...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Leucine aminopeptidase from porcine kidney and the inhibition constant was determined after preincubatin... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50027042 (3-Amino-2-hydroxy-5-methyl-hexanoic acid {2-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Leucine aminopeptidase and the inhibition constant was determined after preincubating the enzyme and in... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50027046 (2-[2-(3-Amino-2-hydroxy-5-methyl-hexanoylamino)-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of aminopeptidase M was determined and Ki* was reported which is obtained by the equatio... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50027046 (2-[2-(3-Amino-2-hydroxy-5-methyl-hexanoylamino)-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against aminopeptidase M | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50027046 (2-[2-(3-Amino-2-hydroxy-5-methyl-hexanoylamino)-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Leucine aminopeptidase and the inhibition constant was determined after preincubating the enzyme and inh... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50017478 (Amastatin | CHEMBL28650 | Leu[1psi,CHOHCONH]ValVal...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of aminopeptidase M was determined and the Ki was reported which is = k2/k1 | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50367209 (BESTATIN HYDROCHLORIDE | Ubenimex) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB PubMed | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Aminopeptidase M (AP-M) and the inhibition constant was determined after preincubating the enzyme and in... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50367209 (BESTATIN HYDROCHLORIDE | Ubenimex) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB PubMed | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of aminopeptidase M was determined and Ki* was reported which is obtained by the equatio... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50367209 (BESTATIN HYDROCHLORIDE | Ubenimex) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of aminopeptidase M was determined and the Ki was reported which is = k2/k1 | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50027041 (2-(3-Amino-2-hydroxy-5-methyl-hexanoylamino)-4-met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Aminopeptidase M (AP-M) and the inhibition constant was determined after preincubating the enzyme and in... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50027043 (3-Amino-2-hydroxy-N-(3-methyl-butyl)-4-phenyl-buty...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Aminopeptidase M (AP-M) from porcine kidney and the inhibition constant was determined after preincubati... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50027043 (3-Amino-2-hydroxy-N-(3-methyl-butyl)-4-phenyl-buty...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of aminopeptidase M was determined and Ki* was reported which is obtained by the equatio... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50027043 (3-Amino-2-hydroxy-N-(3-methyl-butyl)-4-phenyl-buty...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Aminopeptidase M (AP-M) and the inhibition constant was determined after preincubating the enzyme and in... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50046325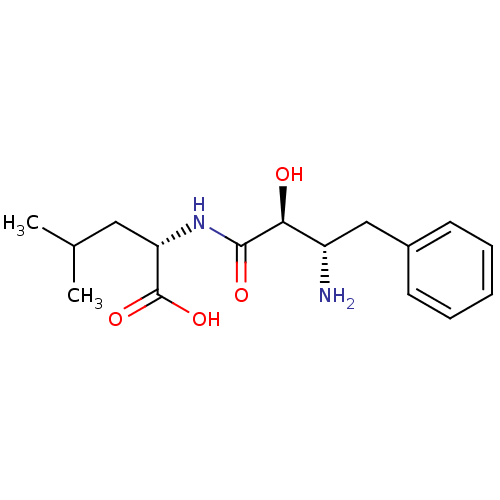 ((S)-2-((2S,3S)-3-Amino-2-hydroxy-4-phenyl-butyryla...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Leucine aminopeptidase and the inhibition constant was determined after preincubating the enzyme and inh... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50027045 (2-{2-[2-(3-Amino-2-hydroxy-5-methyl-hexanoylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of Aminopeptidase M from porcine kidney was determined and the Ki was reported which is ... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50027045 (2-{2-[2-(3-Amino-2-hydroxy-5-methyl-hexanoylamino)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of aminopeptidase M was determined and the Ki was reported which is = k2/k1 | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50017478 (Amastatin | CHEMBL28650 | Leu[1psi,CHOHCONH]ValVal...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | MMDB PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of aminopeptidase M was determined and the Ki was reported which is = k2/k1 | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50367209 (BESTATIN HYDROCHLORIDE | Ubenimex) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Leucine aminopeptidase and the inhibition constant was determined after preincubating the enzyme and inh... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50027045 (2-{2-[2-(3-Amino-2-hydroxy-5-methyl-hexanoylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | <2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Aminopeptidase M (AP-M) and the inhibition constant was determined after preincubating the enzyme and in... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50027040 (2-{2-[2-(3-Amino-5-methyl-hexanoylamino)-3-methyl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Aminopeptidase M (AP-M) and the inhibition constant was determined after preincubating the enzyme and in... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50027045 (2-{2-[2-(3-Amino-2-hydroxy-5-methyl-hexanoylamino)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against aminopeptidase M | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50027046 (2-[2-(3-Amino-2-hydroxy-5-methyl-hexanoylamino)-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of aminopeptidase M was determined and the Ki was reported which is = k2/k1 | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50046325 ((S)-2-((2S,3S)-3-Amino-2-hydroxy-4-phenyl-butyryla...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of aminopeptidase M was determined and Ki* was reported which is obtained by the equatio... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50027045 (2-{2-[2-(3-Amino-2-hydroxy-5-methyl-hexanoylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of Leucine aminopeptidase was determined and the Ki was reported which is = k2/k1 | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50046325 ((S)-2-((2S,3S)-3-Amino-2-hydroxy-4-phenyl-butyryla...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of aminopeptidase M was determined and the Ki was reported which is = k2/k1 | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50027040 (2-{2-[2-(3-Amino-5-methyl-hexanoylamino)-3-methyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 6.81E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Leucine aminopeptidase and the inhibition constant was determined after preincubating the enzyme and inh... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50027044 (2-{2-[2-(2-Hydroxy-5-methyl-hexanoylamino)-3-methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Aminopeptidase M (AP-M) from porcine kidney and the inhibition constant was determined after preincubati... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50027044 (2-{2-[2-(2-Hydroxy-5-methyl-hexanoylamino)-3-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Leucine aminopeptidase from porcine kidney and the inhibition constant was determined after preincubatin... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50027047 (4-Amino-3-hydroxy-6-methyl-heptanoic acid {2-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Leucine aminopeptidase from porcine kidney and the inhibition constant was determined after preincubatin... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50027047 (4-Amino-3-hydroxy-6-methyl-heptanoic acid {2-methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Aminopeptidase M (AP-M) from porcine kidney and the inhibition constant was determined after preincubati... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50027050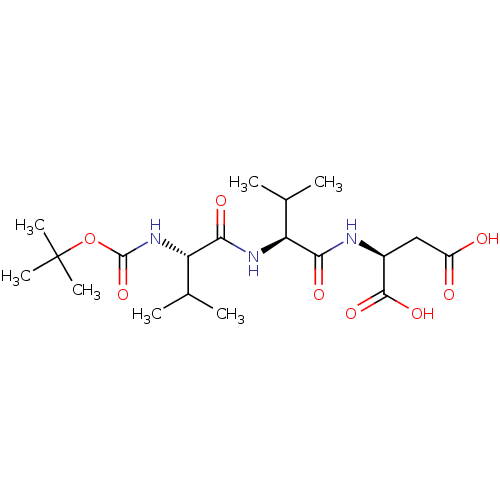 (2-[2-(2-tert-Butoxycarbonylamino-3-methyl-butyryla...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Leucine aminopeptidase from porcine kidney and the inhibition constant was determined after preincubatin... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50027045 (2-{2-[2-(3-Amino-2-hydroxy-5-methyl-hexanoylamino)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Aminopeptidase M (AP-M) and the inhibition constant was determined after preincubating the enzyme and in... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50027049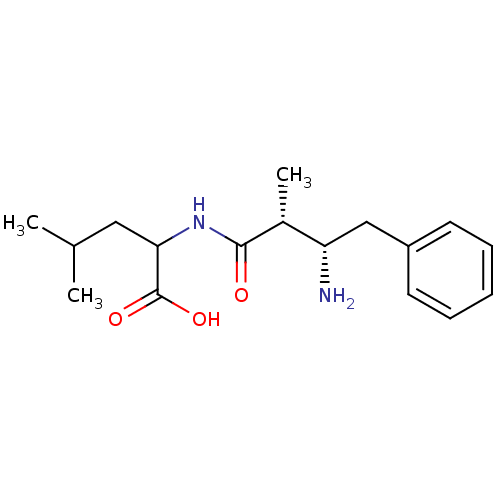 (2-(3-Amino-2-methyl-4-phenyl-butyrylamino)-4-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.78E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Leucine aminopeptidase and the inhibition constant was determined after preincubating the enzyme and inh... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50017478 (Amastatin | CHEMBL28650 | Leu[1psi,CHOHCONH]ValVal...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of Leucine aminopeptidase was determined and Ki* was reported which is obtained by the e... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50367209 (BESTATIN HYDROCHLORIDE | Ubenimex) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 3.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against aminopeptidase M | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50027045 (2-{2-[2-(3-Amino-2-hydroxy-5-methyl-hexanoylamino)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of aminopeptidase M was determined and the Ki was reported which is = k2/k1 | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||