

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V1a receptor (RAT) | BDBM50029649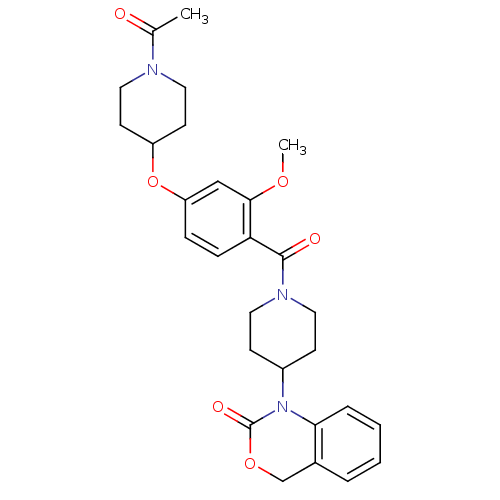 (1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in rat liver | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50029649 (1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-OT (oxytocin) from specific binding sites in uterine tissue obtained from human | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50029646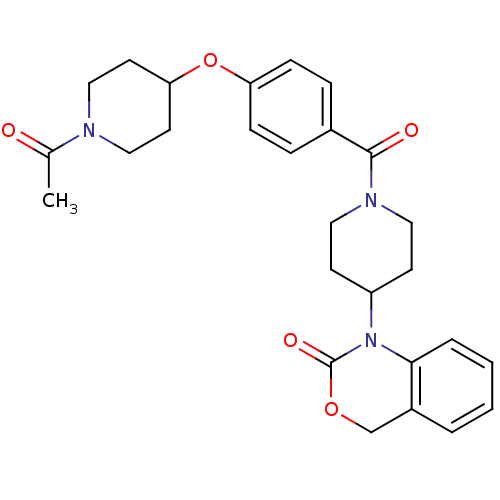 (1-{1-[4-(1-Acetyl-piperidin-4-yloxy)-benzoyl]-pipe...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in rat liver | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50029643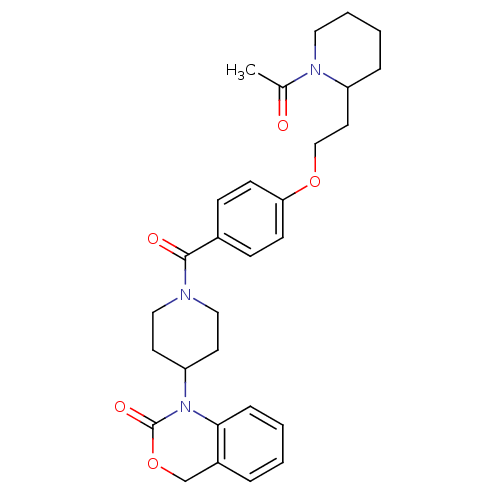 (1-(1-{4-[2-(1-Acetyl-piperidin-2-yl)-ethoxy]-benzo...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in rat liver | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50029649 (1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-OT (oxytocin) from specific binding sites in uterine tissue obtained from rats | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50029646 (1-{1-[4-(1-Acetyl-piperidin-4-yloxy)-benzoyl]-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-OT (oxytocin) from specific binding sites in uterine tissue obtained from human | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50029647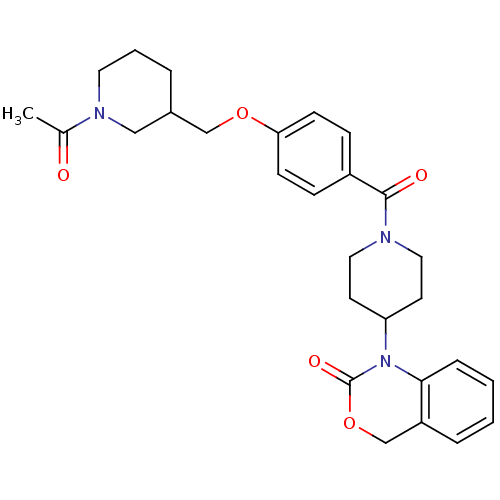 (1-{1-[4-(1-Acetyl-piperidin-3-ylmethoxy)-benzoyl]-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in rat liver | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50029648 (CHEMBL143304 | N-(3-{4-[4-(2-Oxo-4H-benzo[d][1,3]o...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in rat liver. | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50029644 (CHEMBL296908 | N-(3-(4-(4-(2-oxo-3,4-dihydroquinol...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in rat liver. | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50029646 (1-{1-[4-(1-Acetyl-piperidin-4-yloxy)-benzoyl]-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-OT (oxytocin) from specific binding sites in uterine tissue obtained from rats | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50029648 (CHEMBL143304 | N-(3-{4-[4-(2-Oxo-4H-benzo[d][1,3]o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-OT (oxytocin) from specific binding sites in uterine tissue obtained from human ... | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50029643 (1-(1-{4-[2-(1-Acetyl-piperidin-2-yl)-ethoxy]-benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-OT (oxytocin) from specific binding sites in uterine tissue obtained from rats | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50029643 (1-(1-{4-[2-(1-Acetyl-piperidin-2-yl)-ethoxy]-benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-OT (oxytocin) from specific binding sites in uterine tissue obtained from human | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50029644 (CHEMBL296908 | N-(3-(4-(4-(2-oxo-3,4-dihydroquinol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-OT (oxytocin) from specific binding sites in uterine tissue obtained from human ... | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50029647 (1-{1-[4-(1-Acetyl-piperidin-3-ylmethoxy)-benzoyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-OT (oxytocin) from specific binding sites in uterine tissue obtained from human | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50029648 (CHEMBL143304 | N-(3-{4-[4-(2-Oxo-4H-benzo[d][1,3]o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-OT (oxytocin) from specific binding sites in uterine tissue obtained from rats | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50029644 (CHEMBL296908 | N-(3-(4-(4-(2-oxo-3,4-dihydroquinol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-OT (oxytocin) from specific binding sites in uterine tissue obtained from rats | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50029647 (1-{1-[4-(1-Acetyl-piperidin-3-ylmethoxy)-benzoyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-OT (oxytocin) from specific binding sites in uterine tissue obtained from rats | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50029645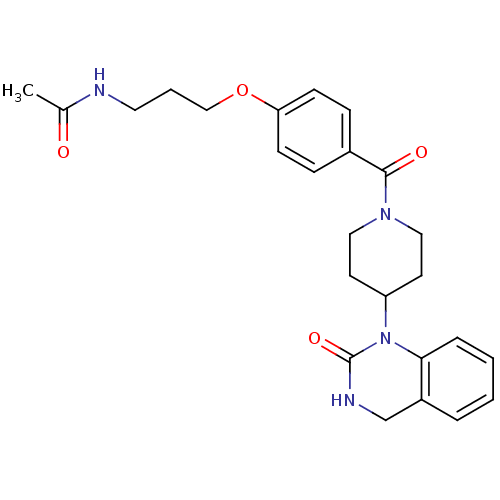 (CHEMBL143341 | N-(3-{4-[4-(2-Oxo-3,4-dihydro-2H-qu...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in rat liver. | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50029645 (CHEMBL143341 | N-(3-{4-[4-(2-Oxo-3,4-dihydro-2H-qu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-OT (oxytocin) from specific binding sites in uterine tissue obtained from human ... | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50029645 (CHEMBL143341 | N-(3-{4-[4-(2-Oxo-3,4-dihydro-2H-qu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-OT (oxytocin) from specific binding sites in uterine tissue obtained from rats | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50029645 (CHEMBL143341 | N-(3-{4-[4-(2-Oxo-3,4-dihydro-2H-qu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in kidney medulla obtaine... | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50029649 (1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in human platelets | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50029647 (1-{1-[4-(1-Acetyl-piperidin-3-ylmethoxy)-benzoyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in kidney medulla obtaine... | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50029643 (1-(1-{4-[2-(1-Acetyl-piperidin-2-yl)-ethoxy]-benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in kidney medulla obtaine... | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50029646 (1-{1-[4-(1-Acetyl-piperidin-4-yloxy)-benzoyl]-pipe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in human platelets | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50029643 (1-(1-{4-[2-(1-Acetyl-piperidin-2-yl)-ethoxy]-benzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in human platelets | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50029649 (1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in kidney medulla obtaine... | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50029644 (CHEMBL296908 | N-(3-(4-(4-(2-oxo-3,4-dihydroquinol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in kidney medulla obtaine... | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50029648 (CHEMBL143304 | N-(3-{4-[4-(2-Oxo-4H-benzo[d][1,3]o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in kidney medulla obtaine... | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50029646 (1-{1-[4-(1-Acetyl-piperidin-4-yloxy)-benzoyl]-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in kidney medulla obtaine... | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50029648 (CHEMBL143304 | N-(3-{4-[4-(2-Oxo-4H-benzo[d][1,3]o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in human platelets. | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50029649 (1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in kidney medulla obtaine... | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50029647 (1-{1-[4-(1-Acetyl-piperidin-3-ylmethoxy)-benzoyl]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in human platelets | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50029644 (CHEMBL296908 | N-(3-(4-(4-(2-oxo-3,4-dihydroquinol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in human platelets. | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50029645 (CHEMBL143341 | N-(3-{4-[4-(2-Oxo-3,4-dihydro-2H-qu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in human platelets. | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50029644 (CHEMBL296908 | N-(3-(4-(4-(2-oxo-3,4-dihydroquinol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in kidney medulla obtaine... | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||