

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50369070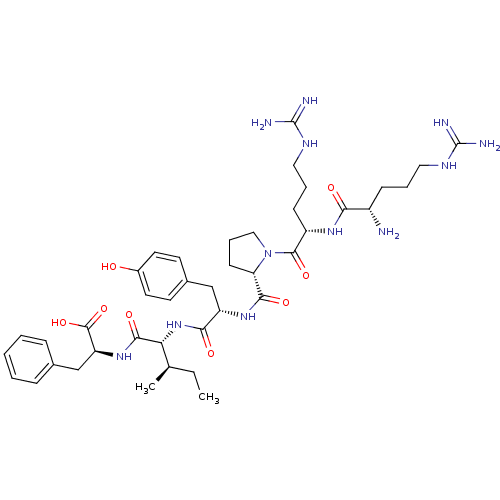 (CHEMBL1793839) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description IC50 was measured as binding to rat cortex membranes using [3H]- NT(neurotensin) as tracer | J Med Chem 38: 1015-21 (1995) BindingDB Entry DOI: 10.7270/Q2DZ08ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50033671 ((S)-2-{(R)-2-[2-({1-[2-(2-Amino-5-guanidino-pentan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description IC50 was measured as binding to rat cortex membranes using [3H]- NT(neurotensin) as tracer | J Med Chem 38: 1015-21 (1995) BindingDB Entry DOI: 10.7270/Q2DZ08ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50240845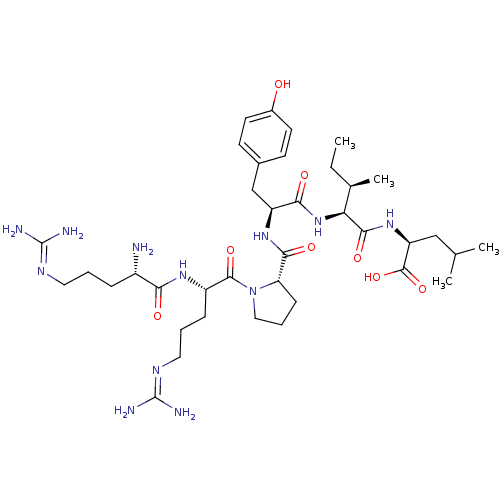 ((S)-2-{(2S,3R)-2-[(S)-2-({(S)-1-[(S)-2-((S)-2-Amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description IC50 was measured as binding to rat cortex membranes using [3H]- NT(neurotensin) as tracer | J Med Chem 38: 1015-21 (1995) BindingDB Entry DOI: 10.7270/Q2DZ08ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50033670 ((S)-2-[(R)-5-({1-[2-(2-Amino-5-guanidino-pentanoyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description IC50 was measured as binding to rat cortex membranes using [3H]- NT(neurotensin) as tracer | J Med Chem 38: 1015-21 (1995) BindingDB Entry DOI: 10.7270/Q2DZ08ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50033677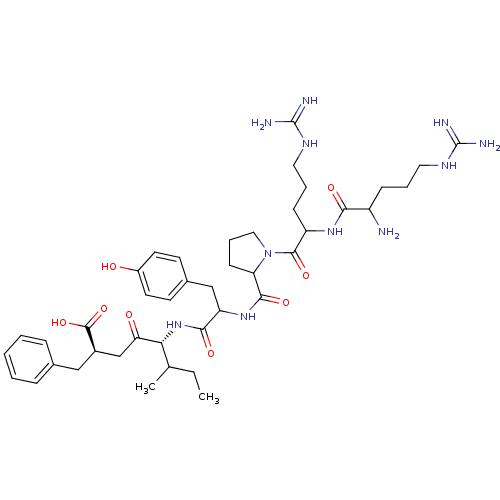 ((S,R)5-[1-{1-[2-[1-amino-4-amino(imino)methylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description IC50 was measured as binding to rat cortex membranes using [3H]- NT(neurotensin) as tracer | J Med Chem 38: 1015-21 (1995) BindingDB Entry DOI: 10.7270/Q2DZ08ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50033667 ((S,S)2-{1-[2-{1-[2-[1-amino-4-amino(imino)methylam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description IC50 was measured as binding to rat cortex membranes using [3H]- NT (neurotensin) as tracer | J Med Chem 38: 1015-21 (1995) BindingDB Entry DOI: 10.7270/Q2DZ08ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50033668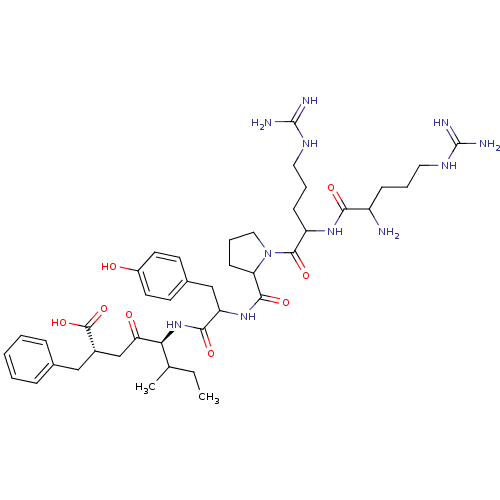 ((R,S)5-[1-{1-[2-[1-amino-4-amino(imino)methylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description IC50 was measured as binding to rat cortex membranes using [3H]- NT(neurotensin) as tracer | J Med Chem 38: 1015-21 (1995) BindingDB Entry DOI: 10.7270/Q2DZ08ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50033675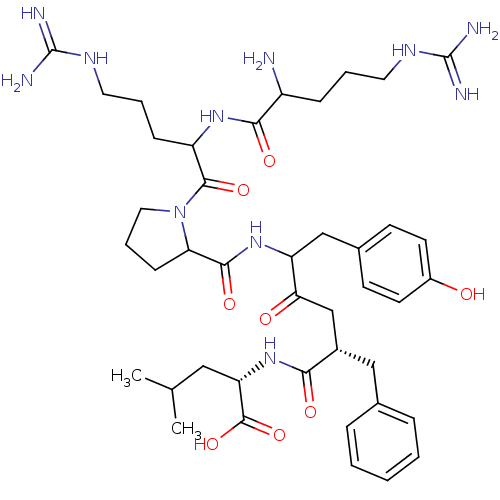 ((S)-2-[(S)-5-({1-[2-(2-Amino-5-guanidino-pentanoyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description IC50 was measured as binding to rat cortex membranes using [3H]- NT(neurotensin) as tracer | J Med Chem 38: 1015-21 (1995) BindingDB Entry DOI: 10.7270/Q2DZ08ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50033678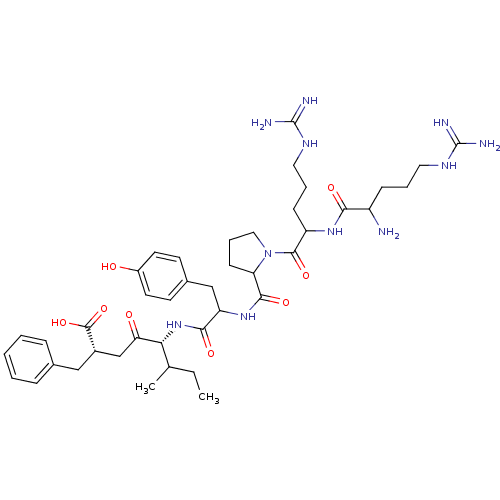 ((S,S)5-[1-{1-[2-[1-amino-4-amino(imino)methylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description IC50 was measured as binding to rat cortex membranes using [3H]- NT(neurotensin) as tracer | J Med Chem 38: 1015-21 (1995) BindingDB Entry DOI: 10.7270/Q2DZ08ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50033679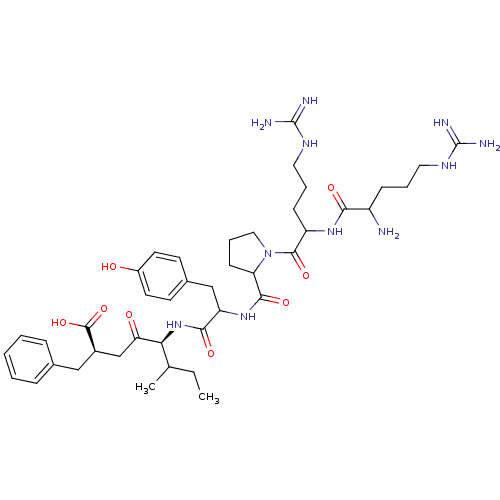 ((R,R)5-[1-{1-[2-[1-amino-4-amino(imino)methylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description IC50 was measured as binding to rat cortex membranes using [3H]- NT(neurotensin) as tracer | J Med Chem 38: 1015-21 (1995) BindingDB Entry DOI: 10.7270/Q2DZ08ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50369071 (CHEMBL1793838) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description IC50 was measured as binding to rat cortex membranes using [3H]- NT(neurotensin) as tracer | J Med Chem 38: 1015-21 (1995) BindingDB Entry DOI: 10.7270/Q2DZ08ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50369072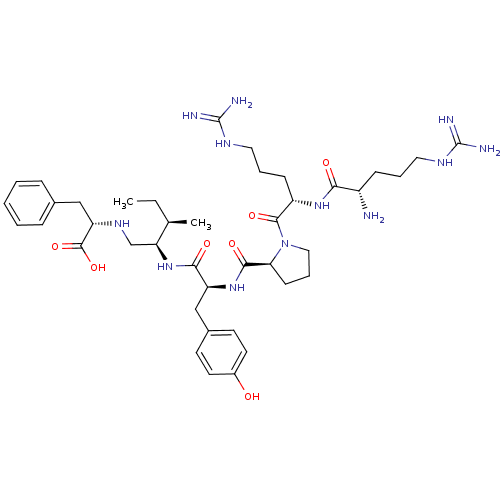 (CHEMBL1793837) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description IC50 was measured as binding to rat cortex membranes using [3H]- NT(neurotensin) as tracer | J Med Chem 38: 1015-21 (1995) BindingDB Entry DOI: 10.7270/Q2DZ08ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50033674 ((S,S)2-{1-[2-{1-[2-[1-amino-4-amino(imino)methylam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description IC50 was measured as binding to rat cortex membranes using [3H]- NT (neurotensin) as tracer | J Med Chem 38: 1015-21 (1995) BindingDB Entry DOI: 10.7270/Q2DZ08ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||