

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50040674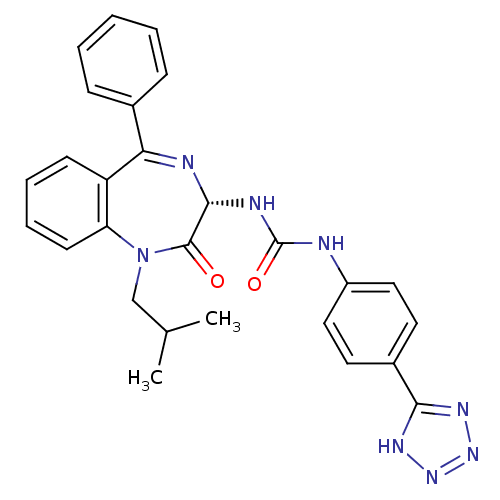 (1-((S)-1-Isobutyl-2-oxo-5-phenyl-2,3-dihydro-1H-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.142 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for binding activity against Cholecystokinin type B receptor from rat pancreatic tissue using [125]BH CCK-8 as radioligand | J Med Chem 37: 722-4 (1994) BindingDB Entry DOI: 10.7270/Q2CN72ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50040678 (1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.266 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for binding activity against Cholecystokinin type B receptor from rat pancreatic tissue using [125]BH CCK-8 as radioligand | J Med Chem 37: 722-4 (1994) BindingDB Entry DOI: 10.7270/Q2CN72ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50040679 (1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.02 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for binding activity against Cholecystokinin type B receptor from rat pancreatic tissue using [125]BH CCK-8 as radioligand | J Med Chem 37: 722-4 (1994) BindingDB Entry DOI: 10.7270/Q2CN72ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50040680 (1-((R)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for binding activity against Cholecystokinin type A receptor from rat pancreas using [125]BH CCK-8s as radioligand. | J Med Chem 37: 722-4 (1994) BindingDB Entry DOI: 10.7270/Q2CN72ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50453549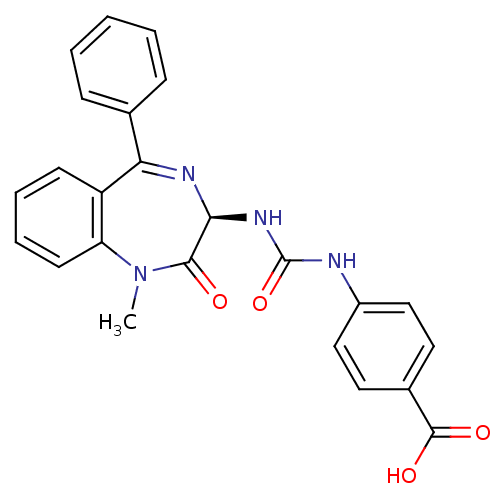 (CHEMBL2112932) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for binding activity against Cholecystokinin type B receptor from rat pancreatic tissue using [125]BH CCK-8 as radioligand | J Med Chem 37: 722-4 (1994) BindingDB Entry DOI: 10.7270/Q2CN72ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50040676 (1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for binding activity against Cholecystokinin type B receptor from rat pancreatic tissue using [125]BH CCK-8 as radioligand | J Med Chem 37: 722-4 (1994) BindingDB Entry DOI: 10.7270/Q2CN72ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50040677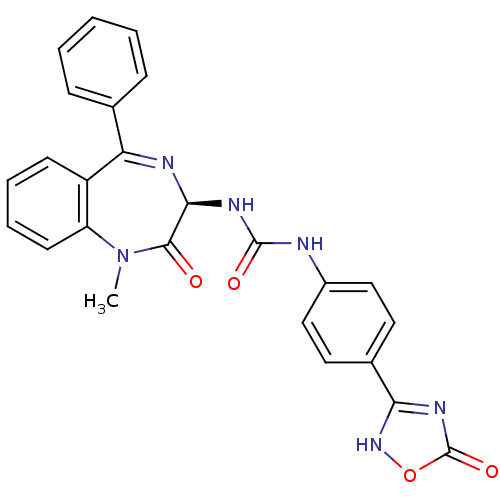 (1-((R)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for binding activity against Cholecystokinin type A receptor from rat pancreas using [125]BH CCK-8s as radioligand. | J Med Chem 37: 722-4 (1994) BindingDB Entry DOI: 10.7270/Q2CN72ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50040675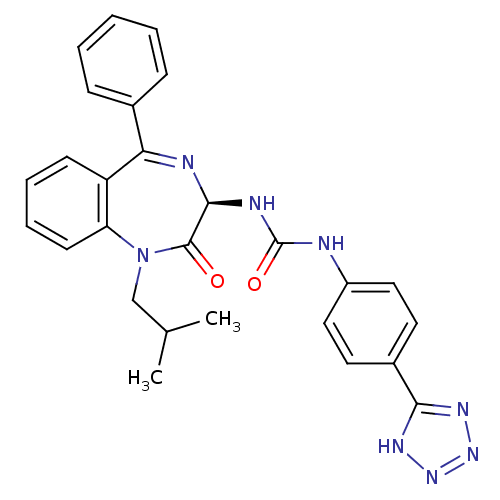 (1-((R)-1-Isobutyl-2-oxo-5-phenyl-2,3-dihydro-1H-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for binding activity against Cholecystokinin type B receptor from rat pancreatic tissue using [125]BH CCK-8 as radioligand | J Med Chem 37: 722-4 (1994) BindingDB Entry DOI: 10.7270/Q2CN72ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50040677 (1-((R)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 56.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for binding activity against Cholecystokinin type B receptor from rat pancreatic tissue using [125]BH CCK-8 as radioligand | J Med Chem 37: 722-4 (1994) BindingDB Entry DOI: 10.7270/Q2CN72ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50040680 (1-((R)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for binding activity against Cholecystokinin type B receptor from rat pancreatic tissue using [125]BH CCK-8 as radioligand | J Med Chem 37: 722-4 (1994) BindingDB Entry DOI: 10.7270/Q2CN72ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50040675 (1-((R)-1-Isobutyl-2-oxo-5-phenyl-2,3-dihydro-1H-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 379 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for binding activity against Cholecystokinin type A receptor from rat pancreas using [125]BH CCK-8s as radioligand. | J Med Chem 37: 722-4 (1994) BindingDB Entry DOI: 10.7270/Q2CN72ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50040679 (1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 577 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for binding activity against Cholecystokinin type A receptor from rat pancreatic tissue using [125]BH CCK-A as radioligand | J Med Chem 37: 722-4 (1994) BindingDB Entry DOI: 10.7270/Q2CN72ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50040676 (1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 736 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for binding activity against Cholecystokinin type A receptor from rat pancreas using [125]BH CCK-8s as radioligand. | J Med Chem 37: 722-4 (1994) BindingDB Entry DOI: 10.7270/Q2CN72ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50040678 (1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 983 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for binding activity against Cholecystokinin type A receptor from rat pancreas using [125]BH CCK-8s as radioligand. | J Med Chem 37: 722-4 (1994) BindingDB Entry DOI: 10.7270/Q2CN72ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50040674 (1-((S)-1-Isobutyl-2-oxo-5-phenyl-2,3-dihydro-1H-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for binding activity against Cholecystokinin type A receptor from rat pancreatic tissue using [125]BH CCK-A as radioligand | J Med Chem 37: 722-4 (1994) BindingDB Entry DOI: 10.7270/Q2CN72ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50453549 (CHEMBL2112932) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for binding activity against Cholecystokinin type A receptor from rat pancreas using [125]BH CCK-8s as radioligand. | J Med Chem 37: 722-4 (1994) BindingDB Entry DOI: 10.7270/Q2CN72ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||