

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50059717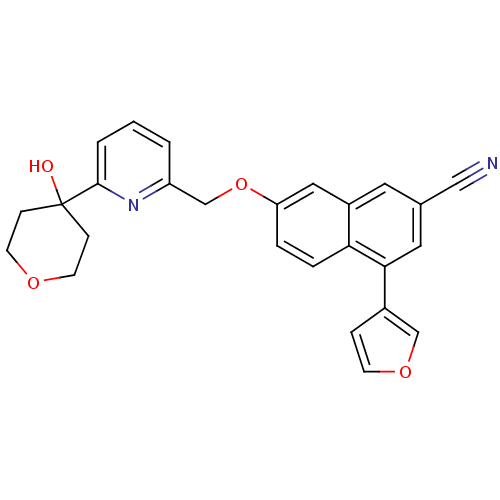 (4-(furan-3-yl)-7-((6-(4-hydroxytetrahydro-2H-pyran...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Compound was evaluated for the potency to inhibit oxidation of arachidonic acid by human recombinant 5-Lipoxygenase using a spectrophotometric assay | J Med Chem 40: 2866-75 (1997) Article DOI: 10.1021/jm970046b BindingDB Entry DOI: 10.7270/Q2QN67G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50059715 (4-Furan-3-yl-7-[6-(3-hydroxy-6,8-dioxa-bicyclo[3.2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of oxidation of arachidonic acid by human 5-Lipoxygenase using spectrophotometric assay | J Med Chem 40: 2866-75 (1997) Article DOI: 10.1021/jm970046b BindingDB Entry DOI: 10.7270/Q2QN67G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053554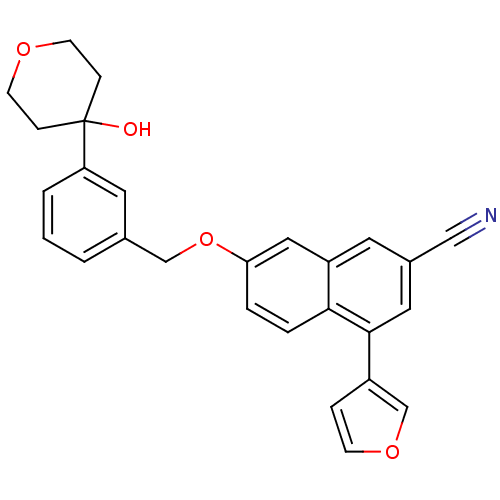 (4-Furan-3-yl-7-[3-(4-hydroxy-tetrahydro-pyran-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of oxidation of arachidonic acid by human 5-Lipoxygenase using spectrophotometric assay | J Med Chem 40: 2866-75 (1997) Article DOI: 10.1021/jm970046b BindingDB Entry DOI: 10.7270/Q2QN67G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50059722 (4-Furan-3-yl-7-[4-(4-hydroxy-tetrahydro-pyran-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of oxidation of arachidonic acid by human 5-Lipoxygenase using spectrophotometric assay | J Med Chem 40: 2866-75 (1997) Article DOI: 10.1021/jm970046b BindingDB Entry DOI: 10.7270/Q2QN67G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50059720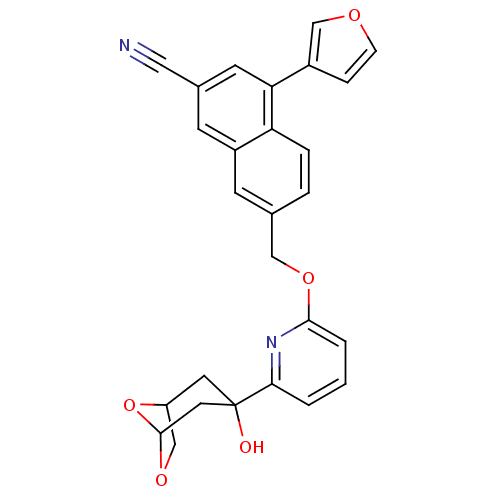 (4-Furan-3-yl-7-[6-(3-hydroxy-6,8-dioxa-bicyclo[3.2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of oxidation of arachidonic acid by human 5-Lipoxygenase using spectrophotometric assay | J Med Chem 40: 2866-75 (1997) Article DOI: 10.1021/jm970046b BindingDB Entry DOI: 10.7270/Q2QN67G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50059714 (4-Furan-3-yl-7-[5-(4-hydroxy-tetrahydro-pyran-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of oxidation of arachidonic acid by human 5-Lipoxygenase using spectrophotometric assay | J Med Chem 40: 2866-75 (1997) Article DOI: 10.1021/jm970046b BindingDB Entry DOI: 10.7270/Q2QN67G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50059719 (4-Furan-3-yl-7-[2-(4-hydroxy-tetrahydro-pyran-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of oxidation of arachidonic acid by human 5-Lipoxygenase using spectrophotometric assay | J Med Chem 40: 2866-75 (1997) Article DOI: 10.1021/jm970046b BindingDB Entry DOI: 10.7270/Q2QN67G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053548 (4-Furan-3-yl-7-[3-(3-hydroxy-6,8-dioxa-bicyclo[3.2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of oxidation of arachidonic acid by human 5-Lipoxygenase using spectrophotometric assay | J Med Chem 40: 2866-75 (1997) Article DOI: 10.1021/jm970046b BindingDB Entry DOI: 10.7270/Q2QN67G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50059721 (4-Furan-3-yl-7-[2-(4-hydroxy-tetrahydro-pyran-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Compound was evaluated for the potency to inhibit oxidation of arachidonic acid by human recombinant 5-Lipoxygenase using a spectrophotometric assay | J Med Chem 40: 2866-75 (1997) Article DOI: 10.1021/jm970046b BindingDB Entry DOI: 10.7270/Q2QN67G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50059718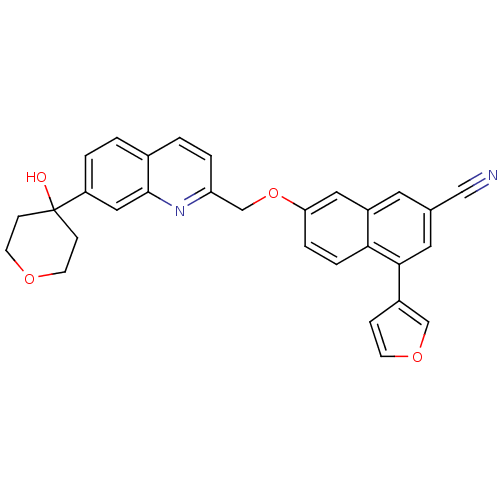 (4-Furan-3-yl-7-[7-(4-hydroxy-tetrahydro-pyran-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of oxidation of arachidonic acid by human 5-Lipoxygenase using spectrophotometric assay | J Med Chem 40: 2866-75 (1997) Article DOI: 10.1021/jm970046b BindingDB Entry DOI: 10.7270/Q2QN67G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50000541 ((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of oxidation of arachidonic acid by human 5-Lipoxygenase using spectrophotometric assay | J Med Chem 40: 2866-75 (1997) Article DOI: 10.1021/jm970046b BindingDB Entry DOI: 10.7270/Q2QN67G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50029559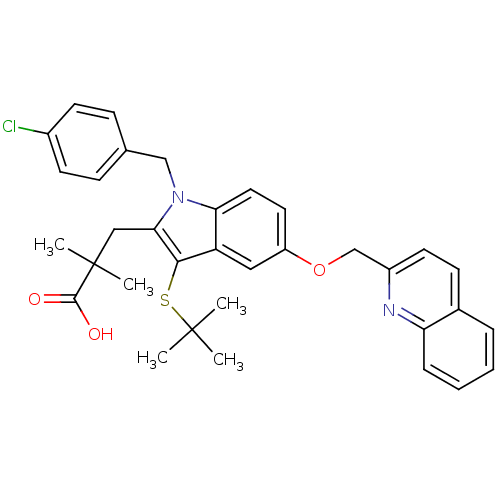 (2-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(qui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of oxidation of arachidonic acid by human 5-Lipoxygenase using spectrophotometric assay | J Med Chem 40: 2866-75 (1997) Article DOI: 10.1021/jm970046b BindingDB Entry DOI: 10.7270/Q2QN67G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||