 Found 54 hits of Enzyme Inhibition Constant Data
Found 54 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50062142

(4,6-Dimethyl-piperidin-(2Z)-ylideneamine | CHEMBL2...)Show InChI InChI=1S/C7H14N2/c1-5-3-6(2)9-7(8)4-5/h5-6H,3-4H2,1-2H3,(H2,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neuronal nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50062142

(4,6-Dimethyl-piperidin-(2Z)-ylideneamine | CHEMBL2...)Show InChI InChI=1S/C7H14N2/c1-5-3-6(2)9-7(8)4-5/h5-6H,3-4H2,1-2H3,(H2,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Inducible nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50062133
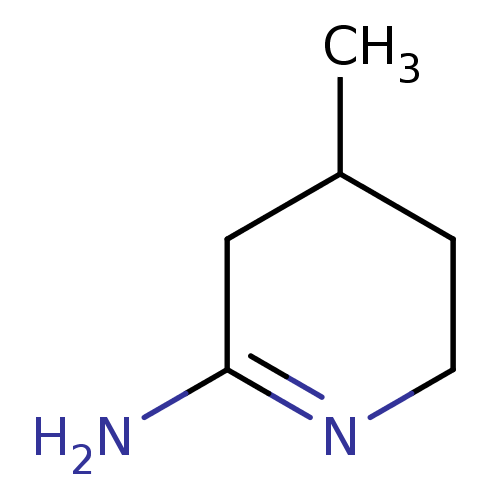
(4-Methyl-piperidin-(2E)-ylideneamine | 4-Methyl-pi...)Show InChI InChI=1S/C6H12N2/c1-5-2-3-8-6(7)4-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Inducible nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50062132
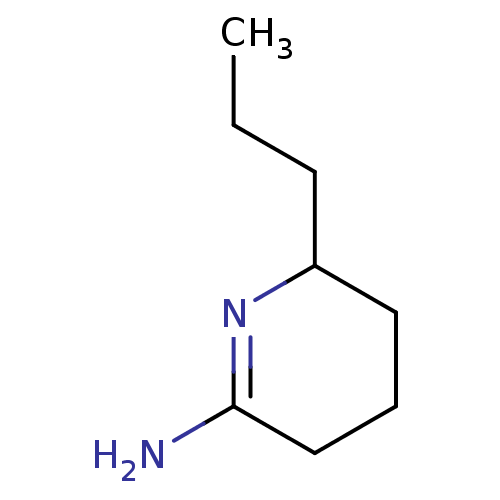
(6-Propyl-piperidin-(2Z)-ylideneamine | CHEMBL6760)Show InChI InChI=1S/C8H16N2/c1-2-4-7-5-3-6-8(9)10-7/h7H,2-6H2,1H3,(H2,9,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Inducible nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50062133

(4-Methyl-piperidin-(2E)-ylideneamine | 4-Methyl-pi...)Show InChI InChI=1S/C6H12N2/c1-5-2-3-8-6(7)4-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neuronal nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50062142

(4,6-Dimethyl-piperidin-(2Z)-ylideneamine | CHEMBL2...)Show InChI InChI=1S/C7H14N2/c1-5-3-6(2)9-7(8)4-5/h5-6H,3-4H2,1-2H3,(H2,8,9) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
inhibition of human endothelial constitutive Endothelial nitric oxide synthase (heNOS) |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50062137
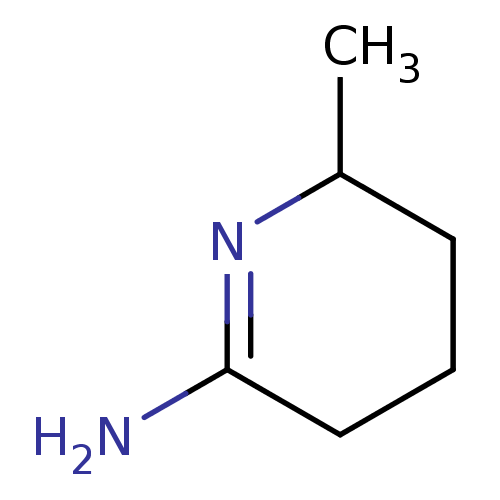
(6-Methyl-piperidin-(2Z)-ylideneamine | CHEMBL26567...)Show InChI InChI=1S/C6H12N2/c1-5-3-2-4-6(7)8-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neuronal nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50062131
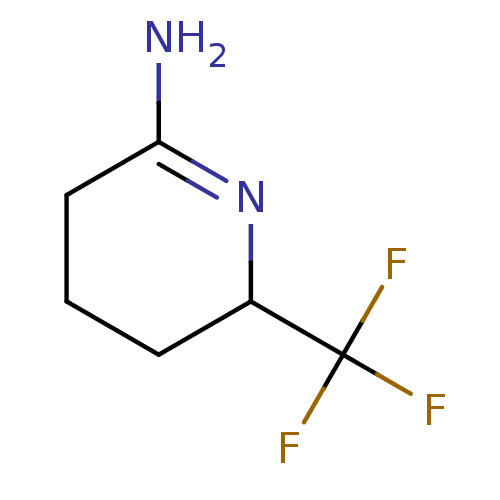
(6-Trifluoromethyl-piperidin-(2Z)-ylideneamine | CH...)Show InChI InChI=1S/C6H9F3N2/c7-6(8,9)4-2-1-3-5(10)11-4/h4H,1-3H2,(H2,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Inducible nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50062137

(6-Methyl-piperidin-(2Z)-ylideneamine | CHEMBL26567...)Show InChI InChI=1S/C6H12N2/c1-5-3-2-4-6(7)8-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Inducible nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50062131

(6-Trifluoromethyl-piperidin-(2Z)-ylideneamine | CH...)Show InChI InChI=1S/C6H9F3N2/c7-6(8,9)4-2-1-3-5(10)11-4/h4H,1-3H2,(H2,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neuronal nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50062132

(6-Propyl-piperidin-(2Z)-ylideneamine | CHEMBL6760)Show InChI InChI=1S/C8H16N2/c1-2-4-7-5-3-6-8(9)10-7/h7H,2-6H2,1H3,(H2,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neuronal nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50062137

(6-Methyl-piperidin-(2Z)-ylideneamine | CHEMBL26567...)Show InChI InChI=1S/C6H12N2/c1-5-3-2-4-6(7)8-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
inhibition of human endothelial constitutive Endothelial nitric oxide synthase (heNOS) |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50062145
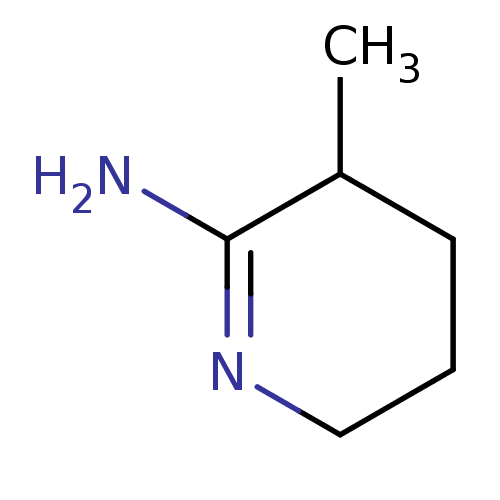
(3-Methyl-piperidin-(2Z)-ylideneamine | CHEMBL26716...)Show InChI InChI=1S/C6H12N2/c1-5-3-2-4-8-6(5)7/h5H,2-4H2,1H3,(H2,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neuronal nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50062136
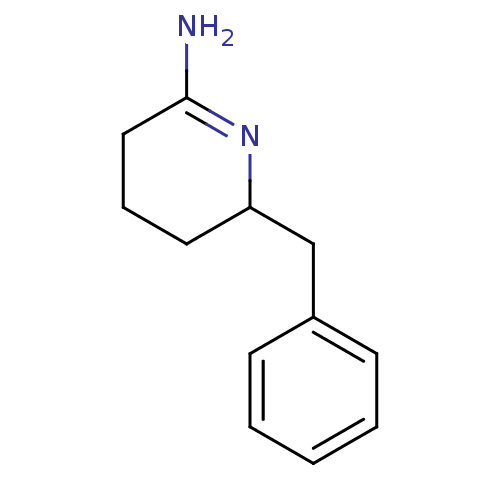
(6-Benzyl-piperidin-(2Z)-ylideneamine | CHEMBL6875)Show InChI InChI=1S/C12H16N2/c13-12-8-4-7-11(14-12)9-10-5-2-1-3-6-10/h1-3,5-6,11H,4,7-9H2,(H2,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neuronal nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50049255
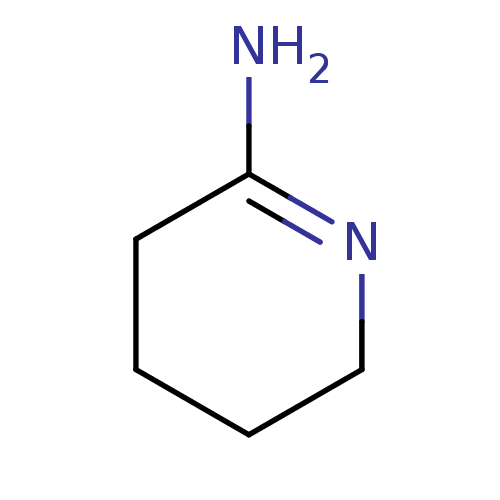
(CHEMBL269058 | PIPERIDIN-2-IMINE | Piperidin-(2E)-...)Show InChI InChI=1S/C5H10N2/c6-5-3-1-2-4-7-5/h1-4H2,(H2,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neuronal nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50049255

(CHEMBL269058 | PIPERIDIN-2-IMINE | Piperidin-(2E)-...)Show InChI InChI=1S/C5H10N2/c6-5-3-1-2-4-7-5/h1-4H2,(H2,6,7) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Inducible nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50062133

(4-Methyl-piperidin-(2E)-ylideneamine | 4-Methyl-pi...)Show InChI InChI=1S/C6H12N2/c1-5-2-3-8-6(7)4-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
inhibition of human endothelial constitutive Endothelial nitric oxide synthase (heNOS) |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50062135

(4-Ethyl-piperidin-(2Z)-ylideneamine | CHEMBL6813)Show InChI InChI=1S/C7H14N2/c1-2-6-3-4-9-7(8)5-6/h6H,2-5H2,1H3,(H2,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neuronal nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50062131

(6-Trifluoromethyl-piperidin-(2Z)-ylideneamine | CH...)Show InChI InChI=1S/C6H9F3N2/c7-6(8,9)4-2-1-3-5(10)11-4/h4H,1-3H2,(H2,10,11) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
inhibition of human endothelial constitutive Endothelial nitric oxide synthase (heNOS) |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50062132

(6-Propyl-piperidin-(2Z)-ylideneamine | CHEMBL6760)Show InChI InChI=1S/C8H16N2/c1-2-4-7-5-3-6-8(9)10-7/h7H,2-6H2,1H3,(H2,9,10) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
inhibition of human endothelial constitutive Endothelial nitric oxide synthase (heNOS) |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50062135

(4-Ethyl-piperidin-(2Z)-ylideneamine | CHEMBL6813)Show InChI InChI=1S/C7H14N2/c1-2-6-3-4-9-7(8)5-6/h6H,2-5H2,1H3,(H2,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Inducible nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50062145

(3-Methyl-piperidin-(2Z)-ylideneamine | CHEMBL26716...)Show InChI InChI=1S/C6H12N2/c1-5-3-2-4-8-6(5)7/h5H,2-4H2,1H3,(H2,7,8) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
inhibition of human endothelial constitutive Endothelial nitric oxide synthase (heNOS) |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50062138
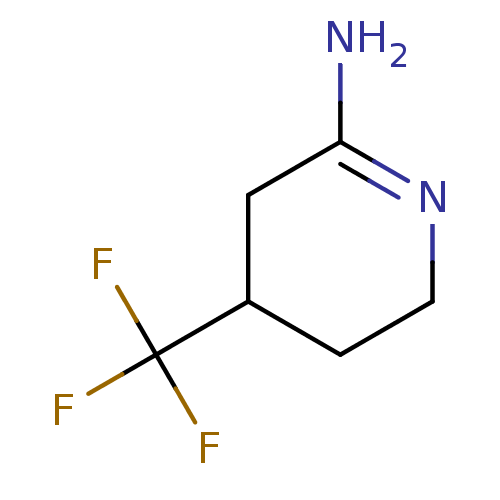
(4-Trifluoromethyl-piperidin-(2Z)-ylideneamine | CH...)Show InChI InChI=1S/C6H9F3N2/c7-6(8,9)4-1-2-11-5(10)3-4/h4H,1-3H2,(H2,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Inducible nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50062138

(4-Trifluoromethyl-piperidin-(2Z)-ylideneamine | CH...)Show InChI InChI=1S/C6H9F3N2/c7-6(8,9)4-1-2-11-5(10)3-4/h4H,1-3H2,(H2,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neuronal nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50062140

(4,4-Dimethyl-piperidin-(2Z)-ylideneamine | CHEMBL6...)Show InChI InChI=1S/C7H14N2/c1-7(2)3-4-9-6(8)5-7/h3-5H2,1-2H3,(H2,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Inducible nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50062139

(5-Methyl-piperidin-(2Z)-ylideneamine | CHEMBL26933...)Show InChI InChI=1S/C6H12N2/c1-5-2-3-6(7)8-4-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neuronal nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50049255

(CHEMBL269058 | PIPERIDIN-2-IMINE | Piperidin-(2E)-...)Show InChI InChI=1S/C5H10N2/c6-5-3-1-2-4-7-5/h1-4H2,(H2,6,7) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
inhibition of human endothelial constitutive Endothelial nitric oxide synthase (heNOS) |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50062139

(5-Methyl-piperidin-(2Z)-ylideneamine | CHEMBL26933...)Show InChI InChI=1S/C6H12N2/c1-5-2-3-6(7)8-4-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Inducible nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50062143
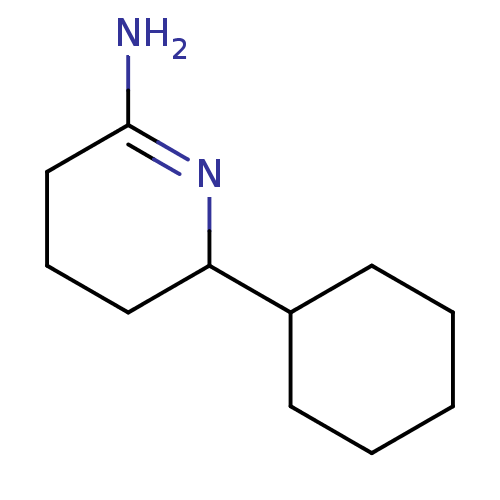
(6-Cyclohexyl-piperidin-(2Z)-ylideneamine | CHEMBL7...)Show InChI InChI=1S/C11H20N2/c12-11-8-4-7-10(13-11)9-5-2-1-3-6-9/h9-10H,1-8H2,(H2,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neuronal nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50062140

(4,4-Dimethyl-piperidin-(2Z)-ylideneamine | CHEMBL6...)Show InChI InChI=1S/C7H14N2/c1-7(2)3-4-9-6(8)5-7/h3-5H2,1-2H3,(H2,8,9) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
inhibition of human endothelial constitutive Endothelial nitric oxide synthase (heNOS) |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50062134
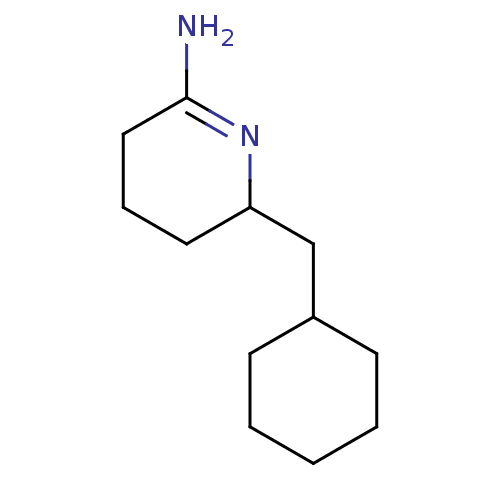
(6-Cyclohexylmethyl-piperidin-(2Z)-ylideneamine | C...)Show InChI InChI=1S/C12H22N2/c13-12-8-4-7-11(14-12)9-10-5-2-1-3-6-10/h10-11H,1-9H2,(H2,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Inducible nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50062140

(4,4-Dimethyl-piperidin-(2Z)-ylideneamine | CHEMBL6...)Show InChI InChI=1S/C7H14N2/c1-7(2)3-4-9-6(8)5-7/h3-5H2,1-2H3,(H2,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neuronal nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50062145

(3-Methyl-piperidin-(2Z)-ylideneamine | CHEMBL26716...)Show InChI InChI=1S/C6H12N2/c1-5-3-2-4-8-6(5)7/h5H,2-4H2,1H3,(H2,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Inducible nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50062143

(6-Cyclohexyl-piperidin-(2Z)-ylideneamine | CHEMBL7...)Show InChI InChI=1S/C11H20N2/c12-11-8-4-7-10(13-11)9-5-2-1-3-6-9/h9-10H,1-8H2,(H2,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Inducible nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50062136

(6-Benzyl-piperidin-(2Z)-ylideneamine | CHEMBL6875)Show InChI InChI=1S/C12H16N2/c13-12-8-4-7-11(14-12)9-10-5-2-1-3-6-10/h1-3,5-6,11H,4,7-9H2,(H2,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Inducible nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50062135

(4-Ethyl-piperidin-(2Z)-ylideneamine | CHEMBL6813)Show InChI InChI=1S/C7H14N2/c1-2-6-3-4-9-7(8)5-6/h6H,2-5H2,1H3,(H2,8,9) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
inhibition of human endothelial constitutive Endothelial nitric oxide synthase (heNOS) |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50062129
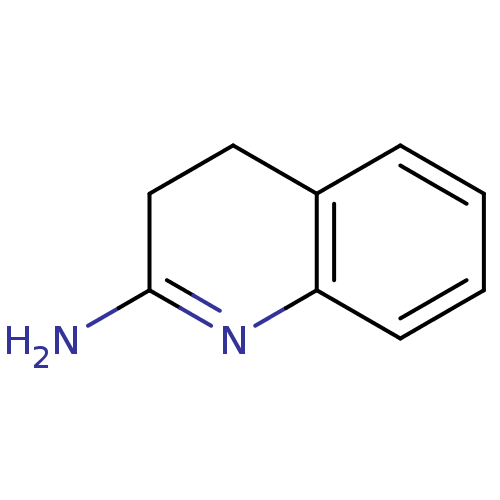
(3,4-Dihydro-1H-quinolin-(2E)-ylideneamine | 3,4-Di...)Show InChI InChI=1S/C9H10N2/c10-9-6-5-7-3-1-2-4-8(7)11-9/h1-4H,5-6H2,(H2,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neuronal nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50062134

(6-Cyclohexylmethyl-piperidin-(2Z)-ylideneamine | C...)Show InChI InChI=1S/C12H22N2/c13-12-8-4-7-11(14-12)9-10-5-2-1-3-6-10/h10-11H,1-9H2,(H2,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neuronal nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50062136

(6-Benzyl-piperidin-(2Z)-ylideneamine | CHEMBL6875)Show InChI InChI=1S/C12H16N2/c13-12-8-4-7-11(14-12)9-10-5-2-1-3-6-10/h1-3,5-6,11H,4,7-9H2,(H2,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
inhibition of human endothelial constitutive Endothelial nitric oxide synthase (heNOS) |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50062138

(4-Trifluoromethyl-piperidin-(2Z)-ylideneamine | CH...)Show InChI InChI=1S/C6H9F3N2/c7-6(8,9)4-1-2-11-5(10)3-4/h4H,1-3H2,(H2,10,11) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
inhibition of human endothelial constitutive Endothelial nitric oxide synthase (heNOS) |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50062139

(5-Methyl-piperidin-(2Z)-ylideneamine | CHEMBL26933...)Show InChI InChI=1S/C6H12N2/c1-5-2-3-6(7)8-4-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
inhibition of human endothelial constitutive Endothelial nitric oxide synthase (heNOS) |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50062129

(3,4-Dihydro-1H-quinolin-(2E)-ylideneamine | 3,4-Di...)Show InChI InChI=1S/C9H10N2/c10-9-6-5-7-3-1-2-4-8(7)11-9/h1-4H,5-6H2,(H2,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Inducible nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50062130

(4-Propyl-piperidin-(2Z)-ylideneamine | CHEMBL6657)Show InChI InChI=1S/C8H16N2/c1-2-3-7-4-5-10-8(9)6-7/h7H,2-6H2,1H3,(H2,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neuronal nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50062144
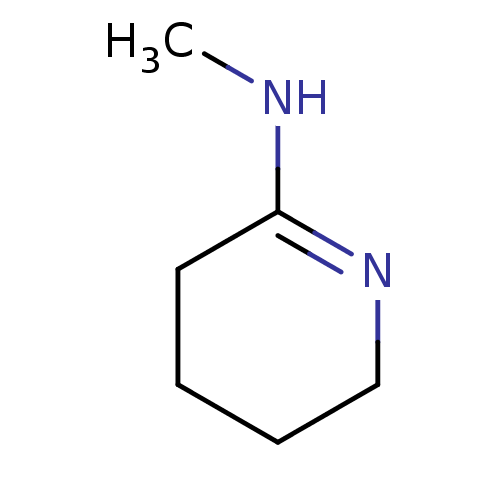
(CHEMBL268294 | Methyl-piperidin-(2Z)-ylidene-amine)Show InChI InChI=1S/C6H12N2/c1-7-6-4-2-3-5-8-6/h2-5H2,1H3,(H,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Inducible nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50062141
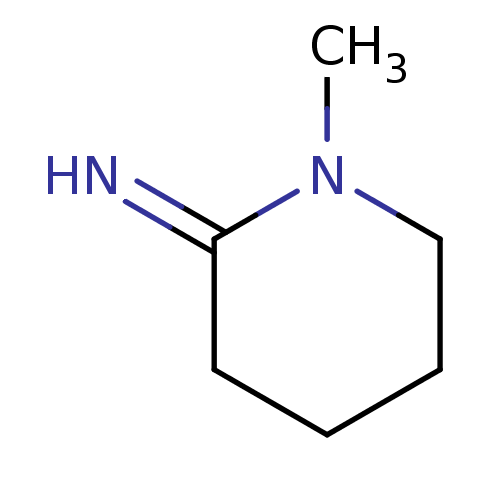
(1-Methyl-piperidin-(2Z)-ylideneamine | CHEMBL26698...)Show InChI InChI=1S/C6H12N2/c1-8-5-3-2-4-6(8)7/h7H,2-5H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
inhibition of human endothelial constitutive Endothelial nitric oxide synthase (heNOS) |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50062130

(4-Propyl-piperidin-(2Z)-ylideneamine | CHEMBL6657)Show InChI InChI=1S/C8H16N2/c1-2-3-7-4-5-10-8(9)6-7/h7H,2-6H2,1H3,(H2,9,10) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
inhibition of human endothelial constitutive Endothelial nitric oxide synthase (heNOS) |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50062144

(CHEMBL268294 | Methyl-piperidin-(2Z)-ylidene-amine)Show InChI InChI=1S/C6H12N2/c1-7-6-4-2-3-5-8-6/h2-5H2,1H3,(H,7,8) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
inhibition of human endothelial constitutive Endothelial nitric oxide synthase (heNOS) |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50062130

(4-Propyl-piperidin-(2Z)-ylideneamine | CHEMBL6657)Show InChI InChI=1S/C8H16N2/c1-2-3-7-4-5-10-8(9)6-7/h7H,2-6H2,1H3,(H2,9,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Inducible nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50062141

(1-Methyl-piperidin-(2Z)-ylideneamine | CHEMBL26698...)Show InChI InChI=1S/C6H12N2/c1-8-5-3-2-4-6(8)7/h7H,2-5H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Inducible nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50062144

(CHEMBL268294 | Methyl-piperidin-(2Z)-ylidene-amine)Show InChI InChI=1S/C6H12N2/c1-7-6-4-2-3-5-8-6/h2-5H2,1H3,(H,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neuronal nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50062141

(1-Methyl-piperidin-(2Z)-ylideneamine | CHEMBL26698...)Show InChI InChI=1S/C6H12N2/c1-8-5-3-2-4-6(8)7/h7H,2-5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Neuronal nitric oxide synthase |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50062143

(6-Cyclohexyl-piperidin-(2Z)-ylideneamine | CHEMBL7...)Show InChI InChI=1S/C11H20N2/c12-11-8-4-7-10(13-11)9-5-2-1-3-6-9/h9-10H,1-8H2,(H2,12,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
inhibition of human endothelial constitutive Endothelial nitric oxide synthase (heNOS) |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50062129

(3,4-Dihydro-1H-quinolin-(2E)-ylideneamine | 3,4-Di...)Show InChI InChI=1S/C9H10N2/c10-9-6-5-7-3-1-2-4-8(7)11-9/h1-4H,5-6H2,(H2,10,11) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
inhibition of human endothelial constitutive Endothelial nitric oxide synthase (heNOS) |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50062134

(6-Cyclohexylmethyl-piperidin-(2Z)-ylideneamine | C...)Show InChI InChI=1S/C12H22N2/c13-12-8-4-7-11(14-12)9-10-5-2-1-3-6-10/h10-11H,1-9H2,(H2,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
inhibition of human endothelial constitutive Endothelial nitric oxide synthase (heNOS) |
J Med Chem 41: 96-101 (1998)
Article DOI: 10.1021/jm9705059
BindingDB Entry DOI: 10.7270/Q2GM86D2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data