

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50069024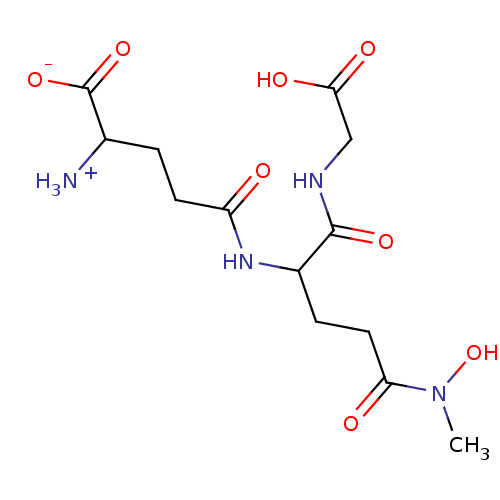 (2-Amino-4-[1-(carboxymethyl-carbamoyl)-3-(hydroxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of S. cerevisiae glyoxalase-I by using enzymatic assay at each of 6 substrate concentrations between 0.1 mM and... | Bioorg Med Chem Lett 8: 705-10 (1999) BindingDB Entry DOI: 10.7270/Q2KH0MGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50241121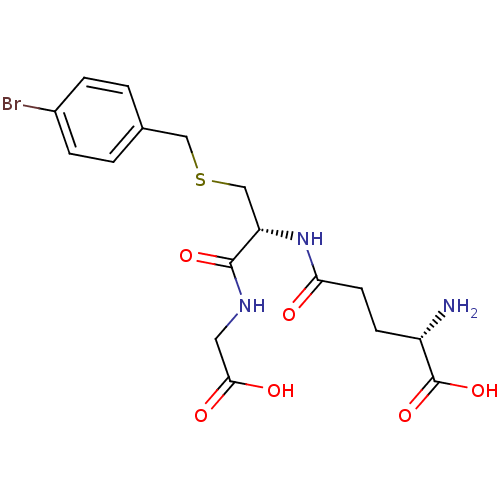 ((S)-5-((R)-3-(4-bromobenzylthio)-1-(carboxymethyla...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of S. cerevisiae glyoxalase-I by using enzymatic assay at each of 6 substrate concentrations between 0.1 mM and... | Bioorg Med Chem Lett 8: 705-10 (1999) BindingDB Entry DOI: 10.7270/Q2KH0MGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50069025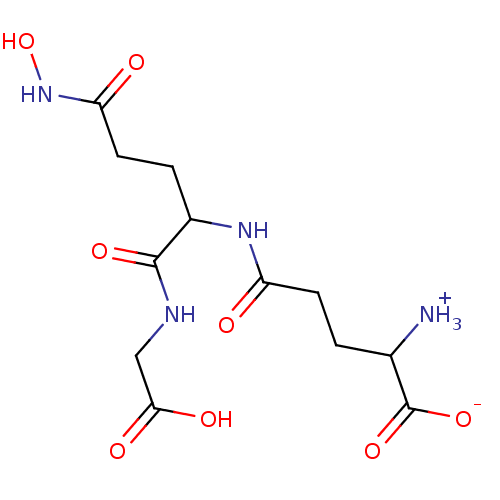 (2-Amino-4-[1-(carboxymethyl-carbamoyl)-3-hydroxyca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Protease was determined | Bioorg Med Chem Lett 8: 705-10 (1999) BindingDB Entry DOI: 10.7270/Q2KH0MGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50069024 (2-Amino-4-[1-(carboxymethyl-carbamoyl)-3-(hydroxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of S. cerevisiae glyoxalase-I, activity is determined with 0.5 mM substrate | Bioorg Med Chem Lett 8: 705-10 (1999) BindingDB Entry DOI: 10.7270/Q2KH0MGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50241121 ((S)-5-((R)-3-(4-bromobenzylthio)-1-(carboxymethyla...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description pA2 against human brain adenosine A1 receptor | Bioorg Med Chem Lett 8: 705-10 (1999) BindingDB Entry DOI: 10.7270/Q2KH0MGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50069026 (2-Amino-4-[1-(ethoxycarbonylmethyl-carbamoyl)-3-(h...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of S. cerevisiae glyoxalase-I, activity is determined with 0.5 mM substrate | Bioorg Med Chem Lett 8: 705-10 (1999) BindingDB Entry DOI: 10.7270/Q2KH0MGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50069025 (2-Amino-4-[1-(carboxymethyl-carbamoyl)-3-hydroxyca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of S. cerevisiae glyoxalase-I, activity is determined with 0.5 mM substrate | Bioorg Med Chem Lett 8: 705-10 (1999) BindingDB Entry DOI: 10.7270/Q2KH0MGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50069027 (2-Amino-4-[1-(ethoxycarbonylmethyl-carbamoyl)-3-hy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.62E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of S. cerevisiae glyoxalase-I, activity i determined with 0.5 mM substrate | Bioorg Med Chem Lett 8: 705-10 (1999) BindingDB Entry DOI: 10.7270/Q2KH0MGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||