

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120578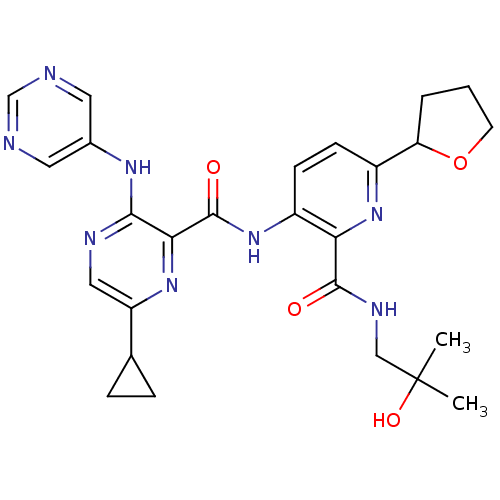 (US8703768, 178) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120577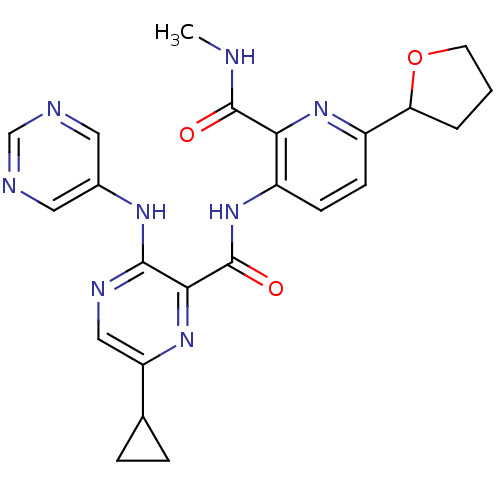 (US8703768, 177) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120643 (US8703768, 244) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120698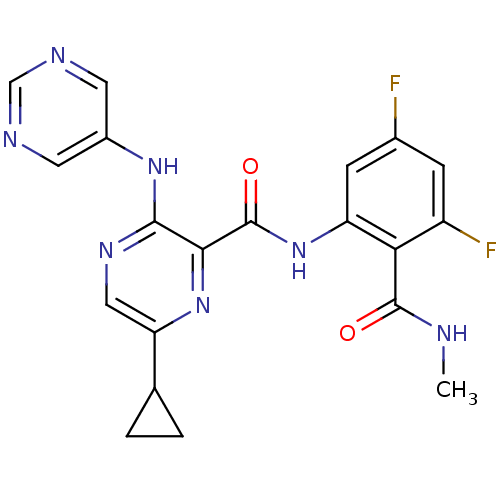 (Roche-Dataset for PDE10A, Compound 927 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120583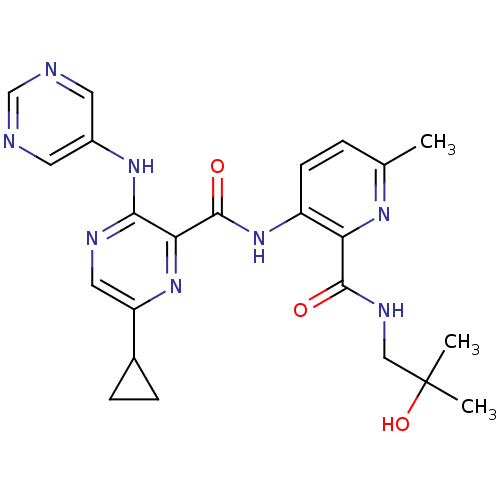 (Roche-Dataset for PDE10A, Compound 707 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120672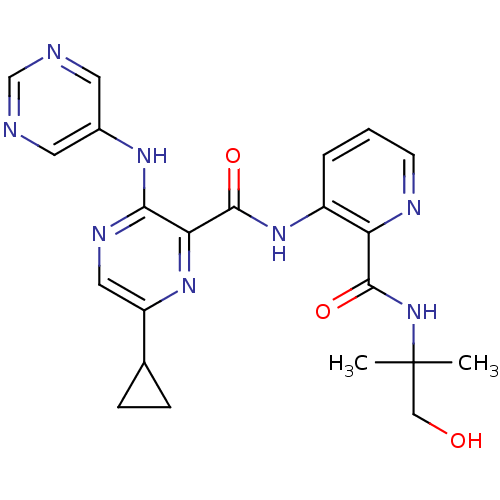 (Roche-Dataset for PDE10A, Compound 803 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120520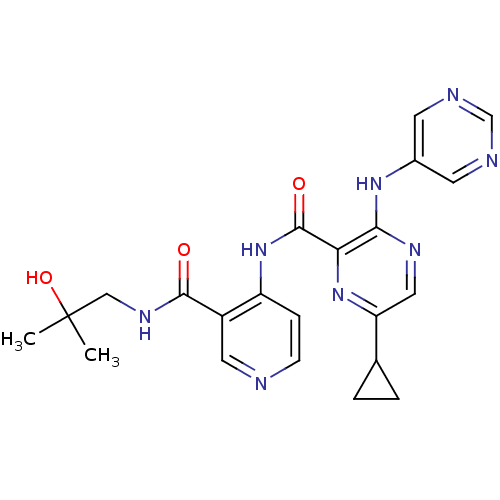 (US8703768, 120) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120481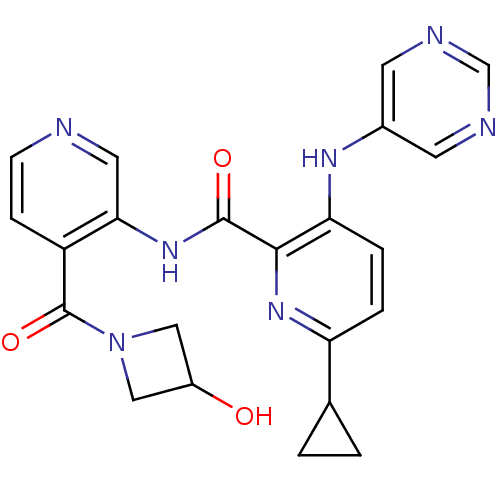 (Roche-Dataset for PDE10A, Compound 503 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120581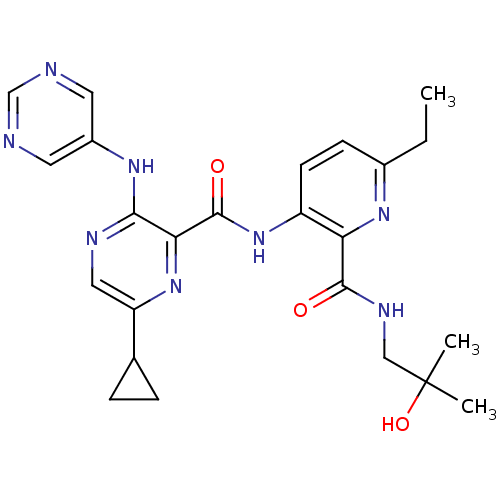 (Roche-Dataset for PDE10A, Compound 553 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120673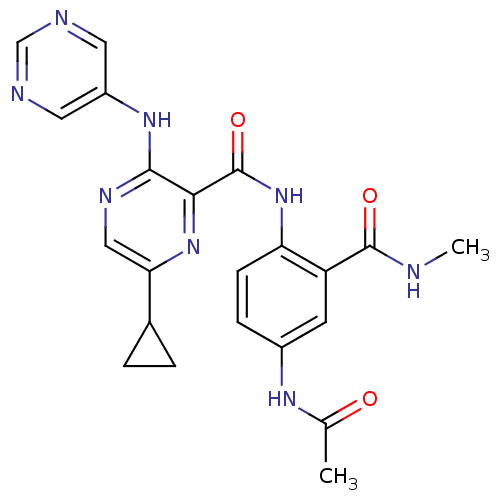 (Roche-Dataset for PDE10A, Compound 599 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120670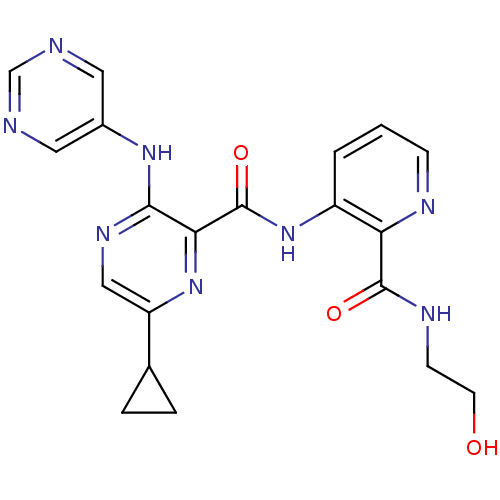 (Roche-Dataset for PDE10A, Compound 852 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120674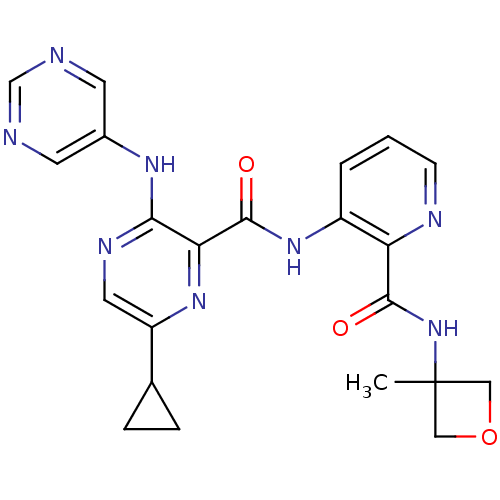 (Roche-Dataset for PDE10A, Compound 770 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120700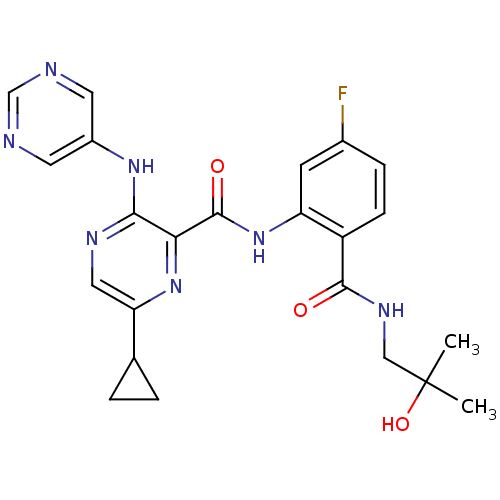 (Roche-Dataset for PDE10A, Compound 902 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120559 (US8703768, 159) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120727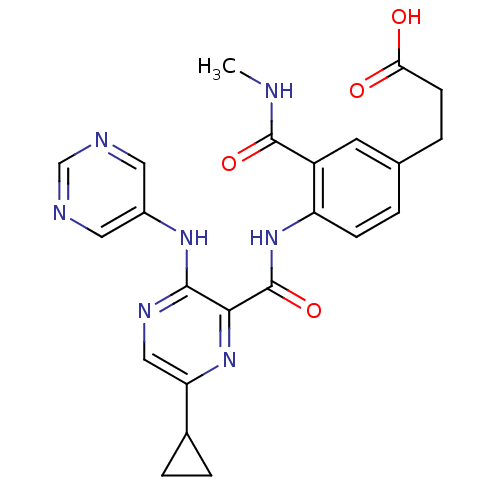 (Roche-Dataset for PDE10A, Compound 481 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120490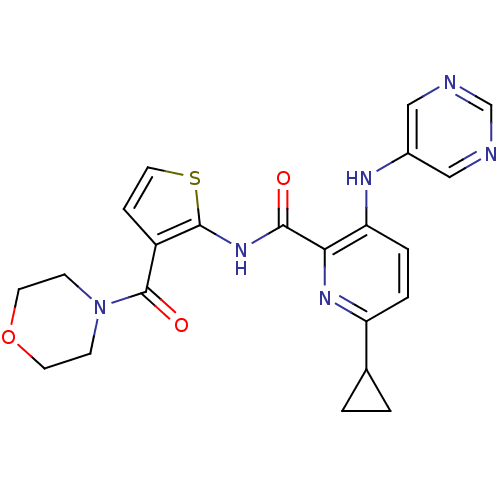 (Roche-Dataset for PDE10A, Compound 743 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120498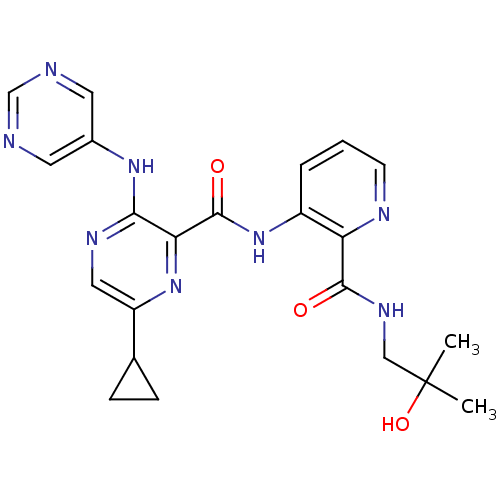 (US8703768, 98) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120489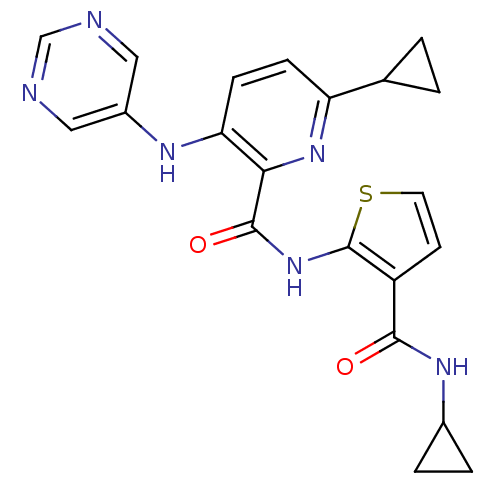 (Roche-Dataset for PDE10A, Compound 997 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120624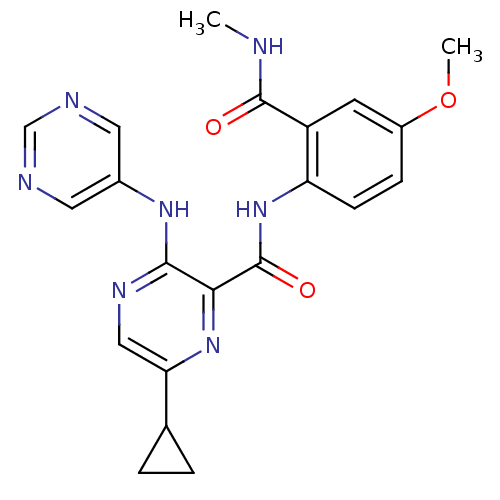 (Roche-Dataset for PDE10A, Compound 933 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120609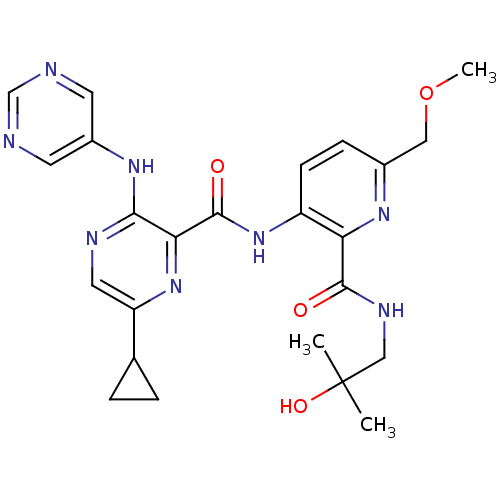 (Roche-Dataset for PDE10A, Compound 539 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120614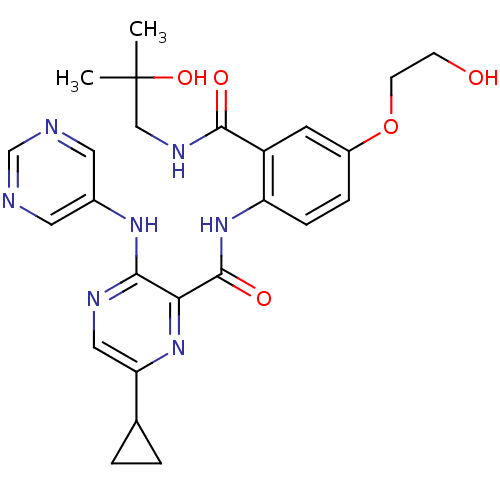 (Roche-Dataset for PDE10A, Compound 464 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120680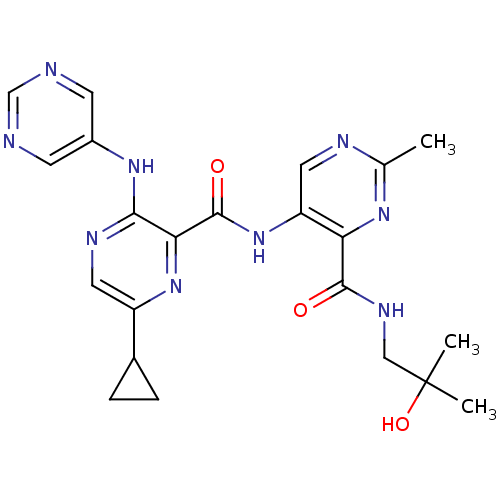 (Roche-Dataset for PDE10A, Compound 798 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120510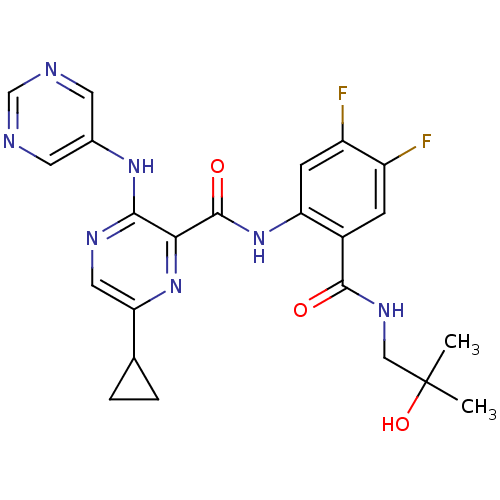 (US8703768, 110) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120720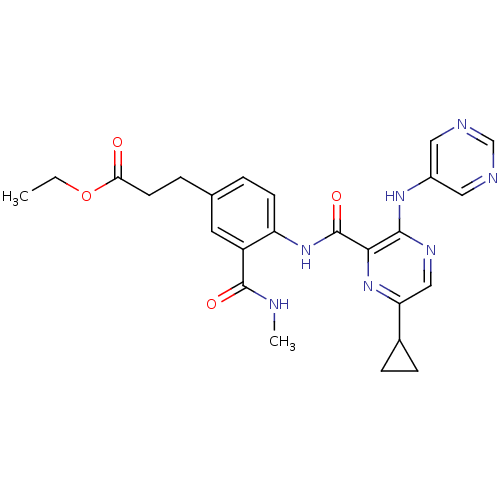 (Roche-Dataset for PDE10A, Compound 329 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120691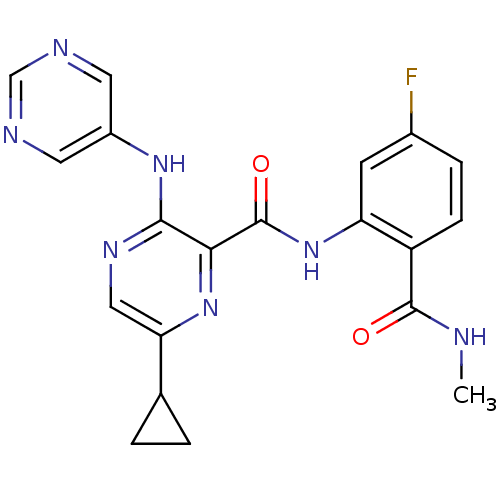 (Roche-Dataset for PDE10A, Compound 913 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120612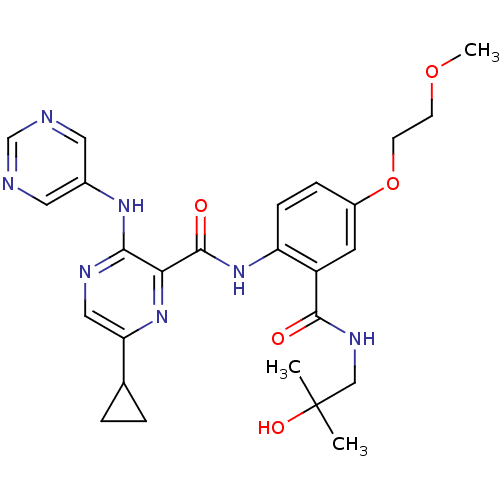 (Roche-Dataset for PDE10A, Compound 323 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120661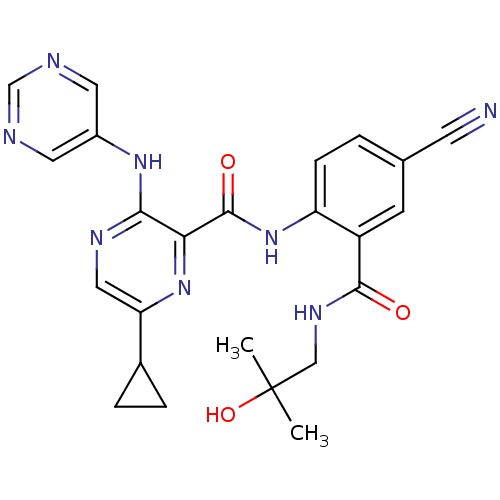 (Roche-Dataset for PDE10A, Compound 708 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120734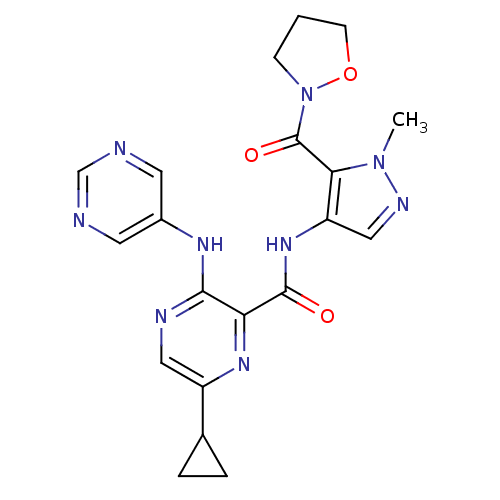 (Roche-Dataset for PDE10A, Compound 740 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120711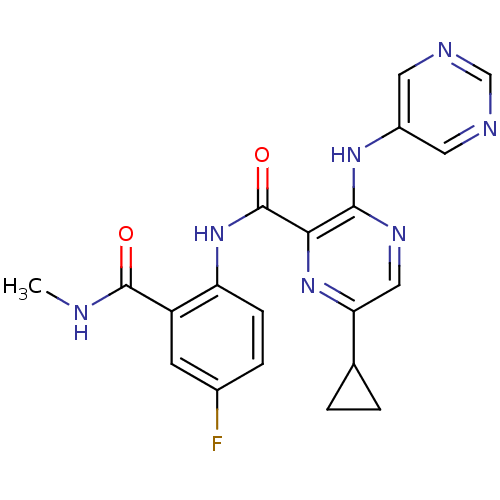 (Roche-Dataset for PDE10A, Compound 922 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120558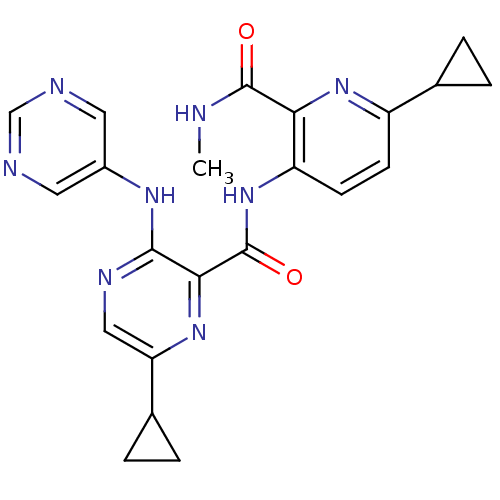 (US8703768, 158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120615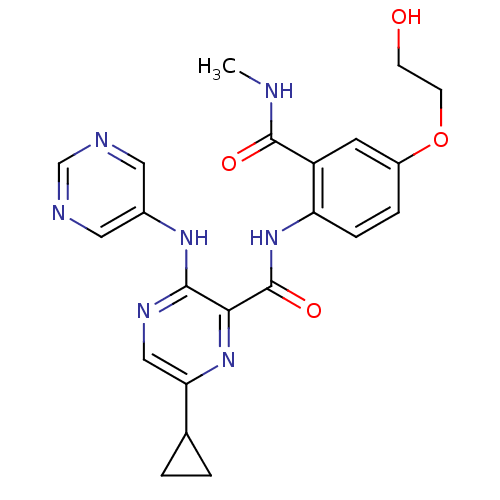 (Roche-Dataset for PDE10A, Compound 527 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120687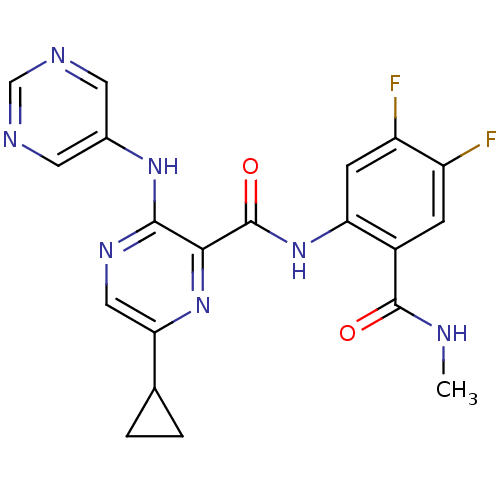 (Roche-Dataset for PDE10A, Compound 912 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120707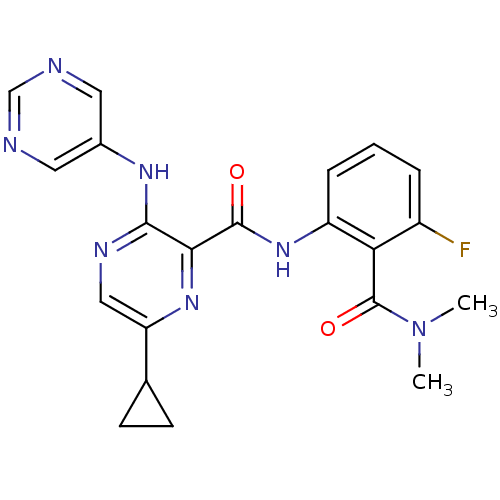 (Roche-Dataset for PDE10A, Compound 702 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120613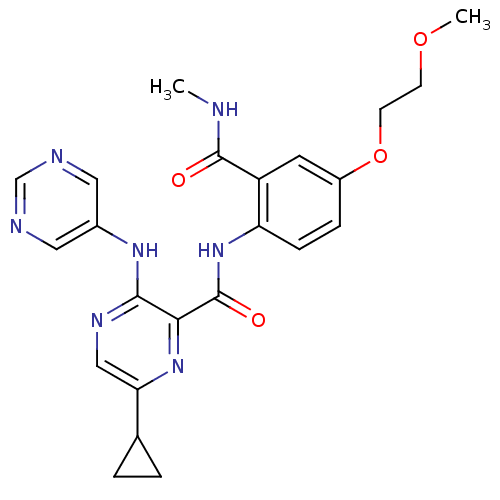 (Roche-Dataset for PDE10A, Compound 536 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120701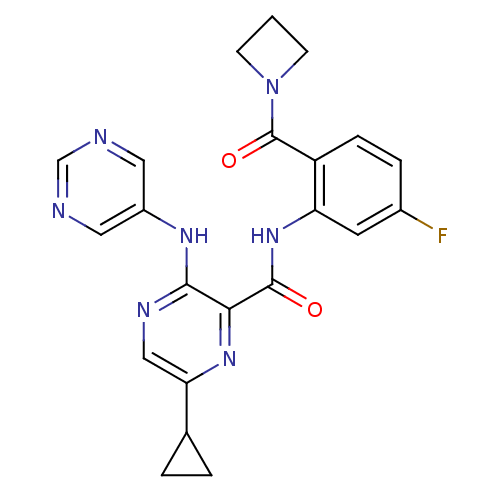 (Roche-Dataset for PDE10A, Compound 755 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120723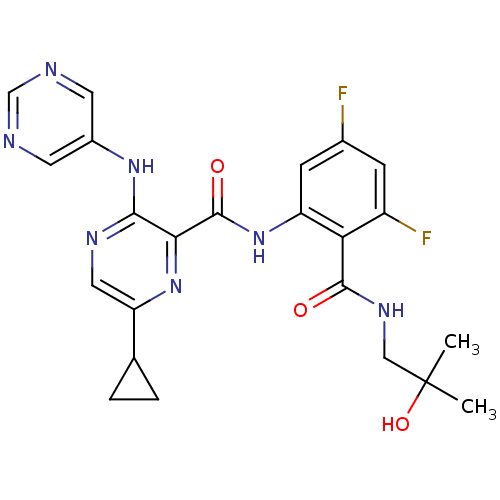 (Roche-Dataset for PDE10A, Compound 756 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120606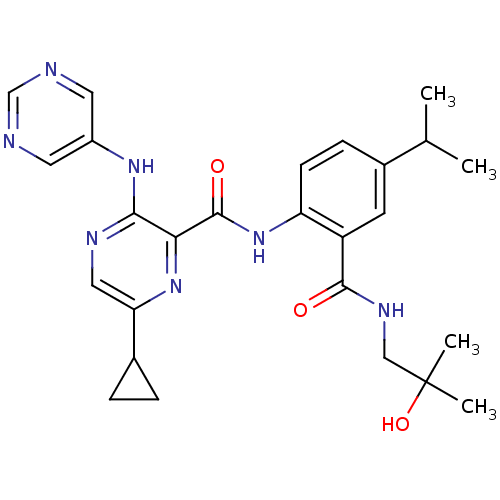 (Roche-Dataset for PDE10A, Compound 494 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120682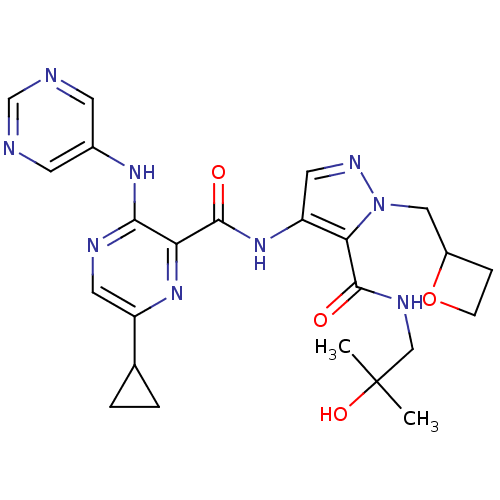 (US8703768, 283) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120514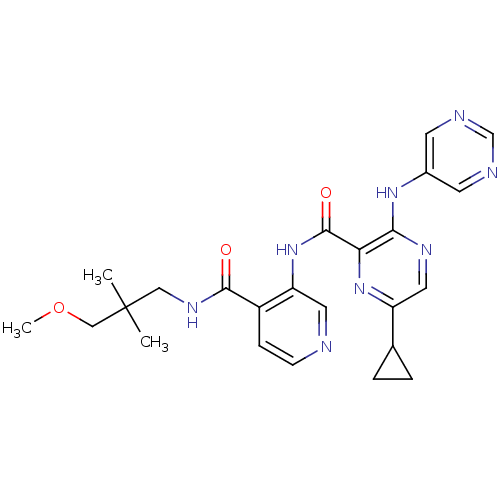 (US8703768, 114) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120505 (US8703768, 105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120708 (Roche-Dataset for PDE10A, Compound 585 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120542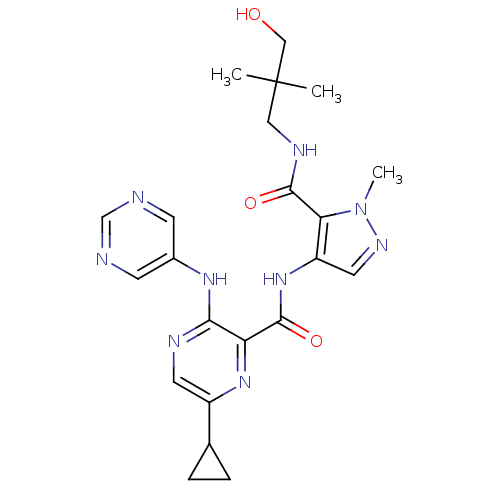 (US8703768, 142) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120729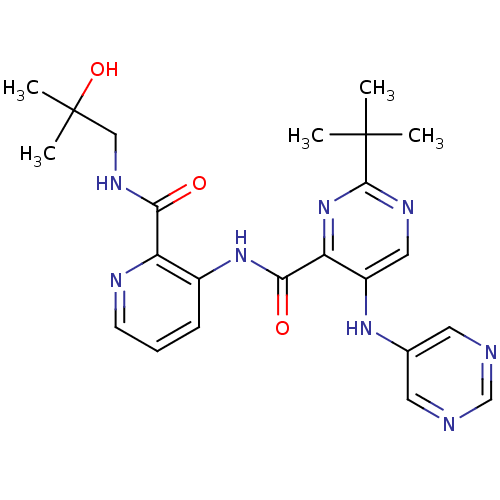 (US8703768, 330) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120458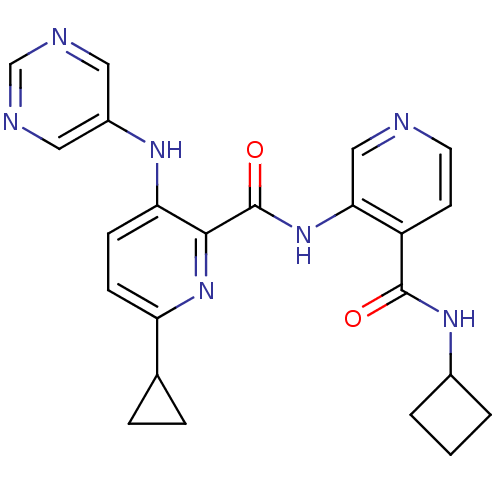 (Roche-Dataset for PDE10A, Compound 564 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120497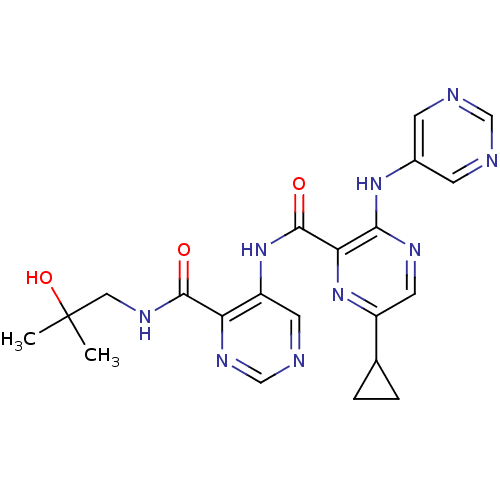 (US8703768, 97) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120547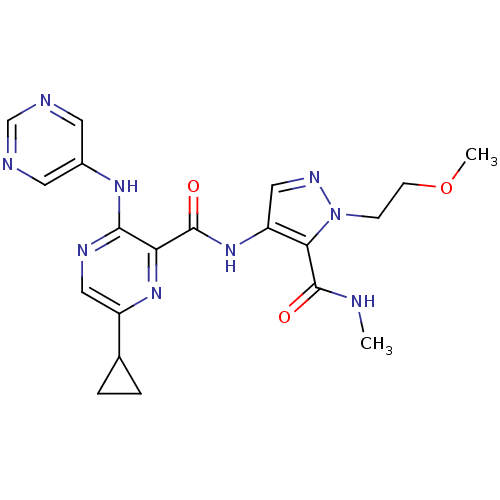 (US8703768, 147) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120412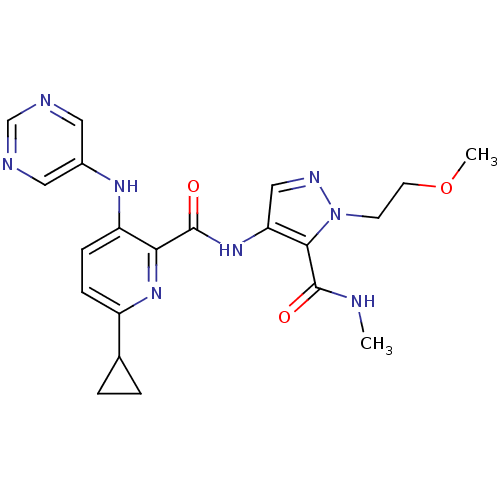 (Roche-Dataset for PDE10A, Compound 1047 | US870376...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120607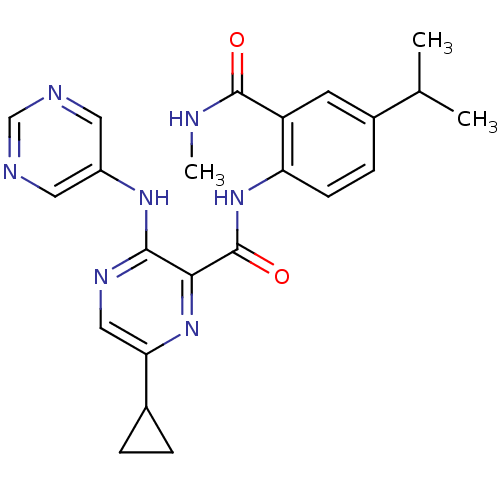 (Roche-Dataset for PDE10A, Compound 766 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120429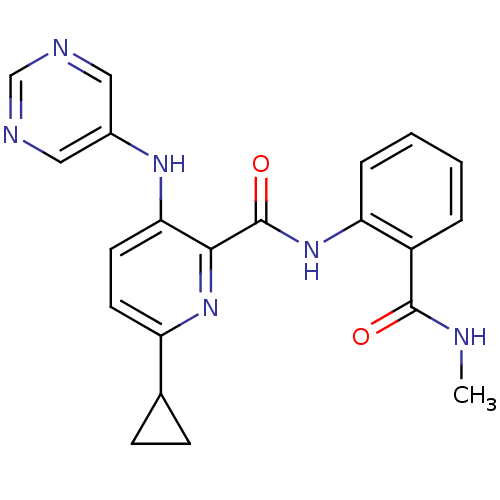 (Roche-Dataset for PDE10A, Compound 969 | US8703768...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM120528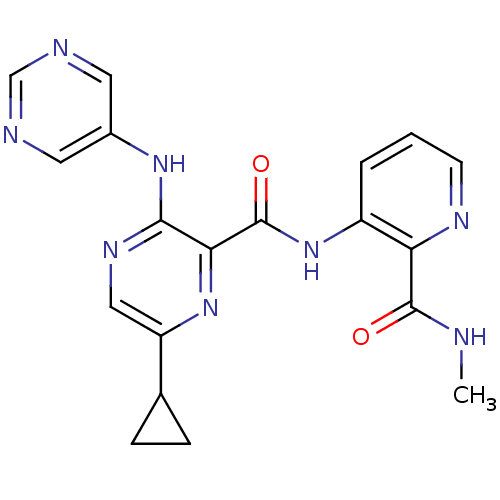 (US8703768, 128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8703768 (2014) BindingDB Entry DOI: 10.7270/Q27P8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 340 total ) | Next | Last >> |