

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM264419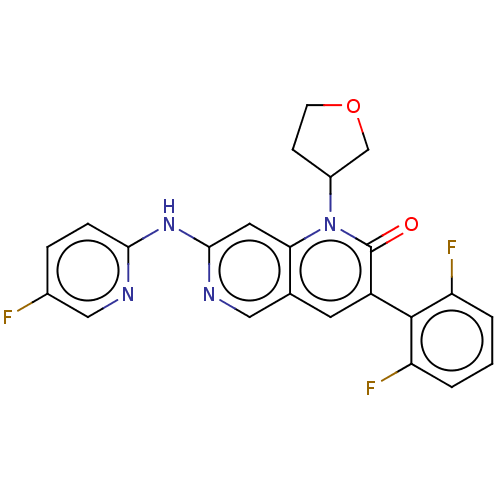 (US9714247, 141 | US9714247, 142) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264421 (US9714247, 143 | US9714247, 144) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264294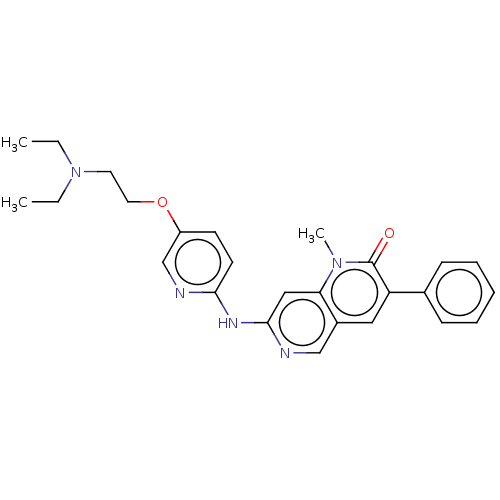 (US9714247, 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM264322 (US9714247, 43) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM264334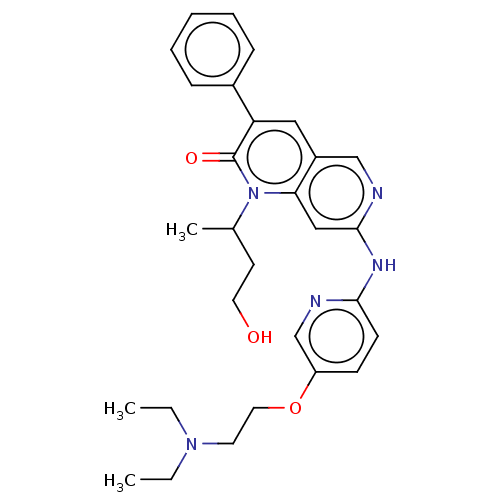 (US9714247, 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM264342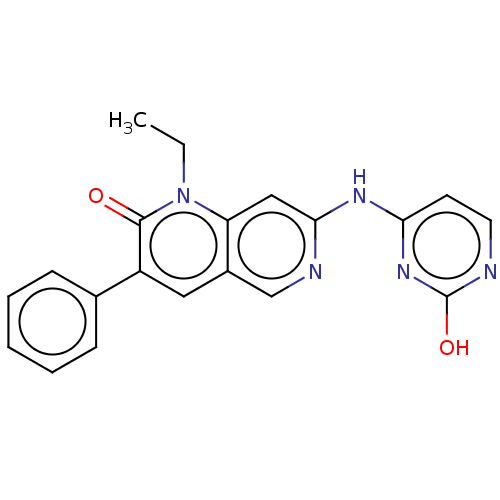 (US9714247, 63) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM264362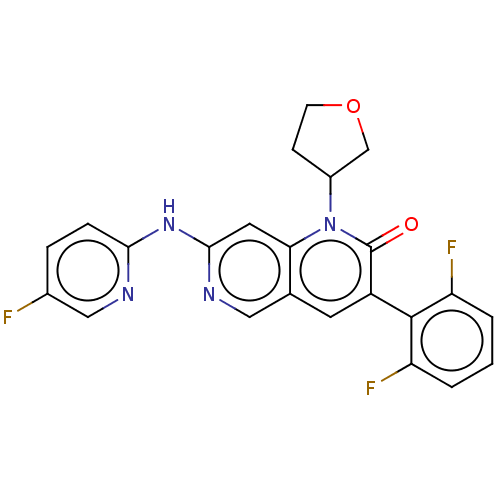 (US9714247, 83) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264372 (US9714247, 93) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264374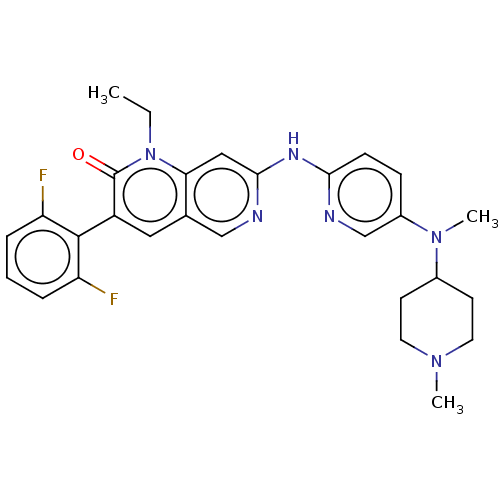 (US9714247, 95) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM264375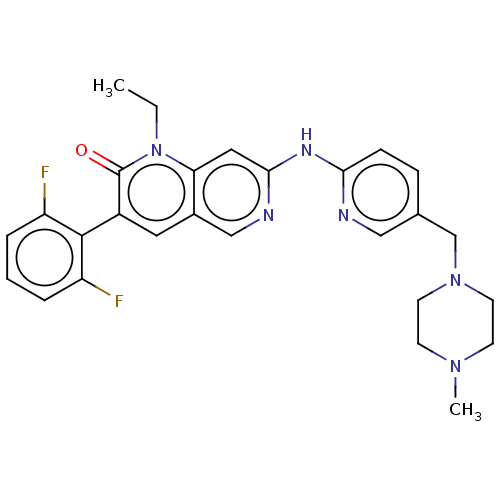 (US9714247, 96) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264379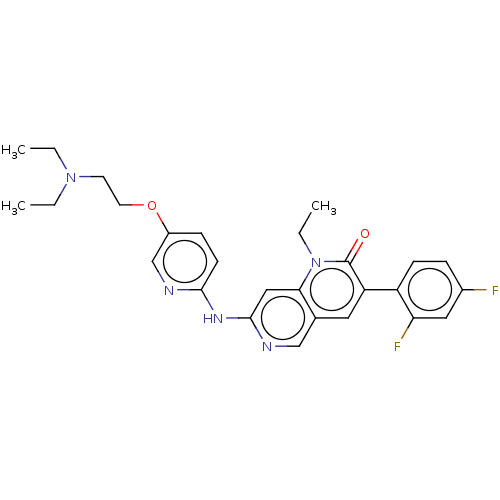 (US9714247, 100) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM264379 (US9714247, 100) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264380 (US9714247, 101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM264382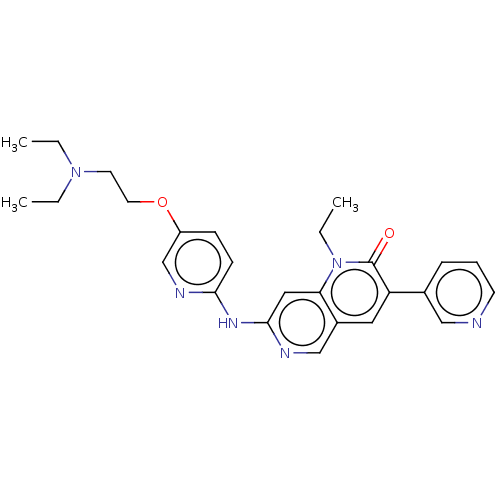 (US9714247, 103) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM264387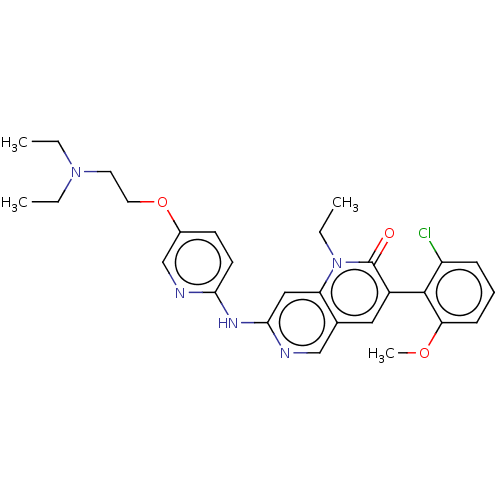 (US9714247, 108) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264390 (US9714247, 111) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM264395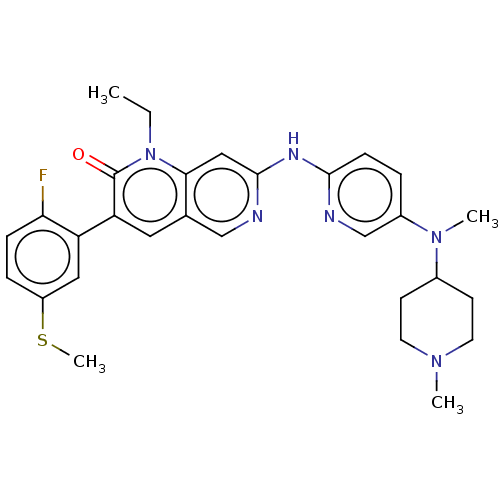 (US9714247, 117) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM264400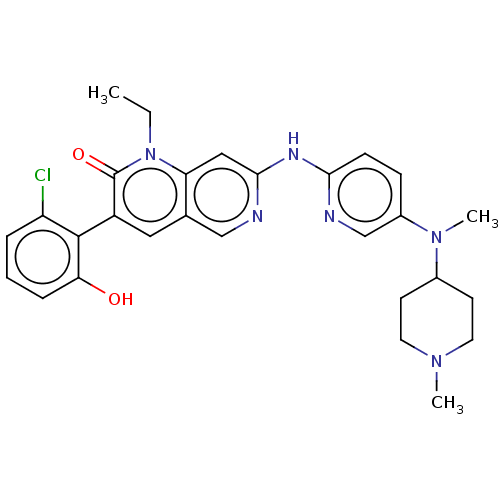 (US9714247, 122) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264408 (US9714247, 130) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM264411 (US9714247, 133) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264412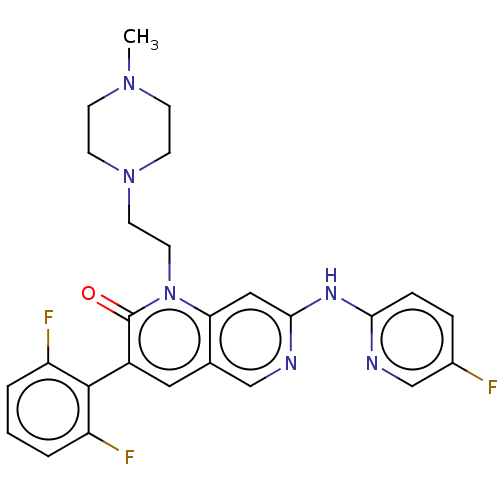 (US9714247, 134) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM264412 (US9714247, 134) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM264414 (US9714247, 136) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM264416 (US9714247, 138) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM264417 (US9714247, 139) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264419 (US9714247, 141 | US9714247, 142) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM264421 (US9714247, 143 | US9714247, 144) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264423 (US9714247, 145 | US9714247, 146) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264293 (US9714247, 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264302 (US9714247, 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264304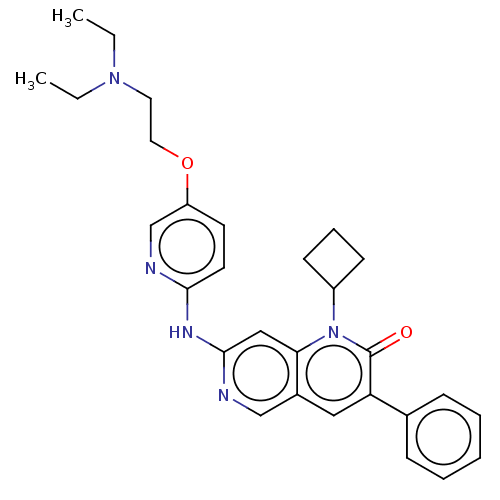 (US9714247, 25) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM264313 (US9714247, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM264314 (US9714247, 35) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM264346 (US9714247, 67) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM264348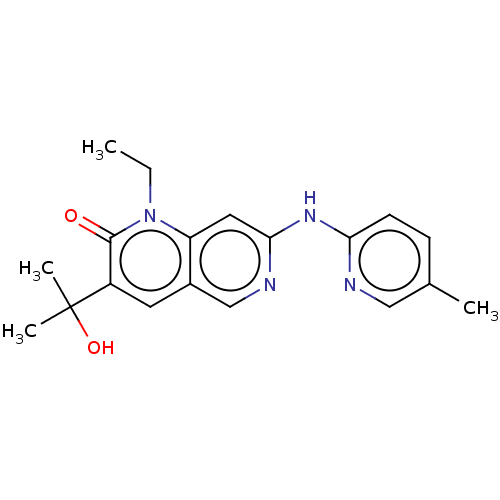 (US9714247, 69) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM264350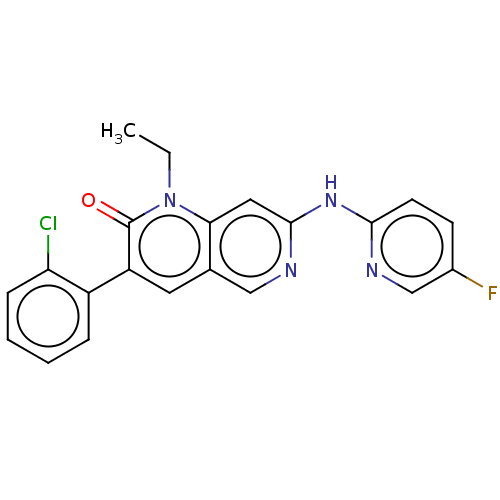 (US9714247, 71) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM264365 (US9714247, 86) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM264376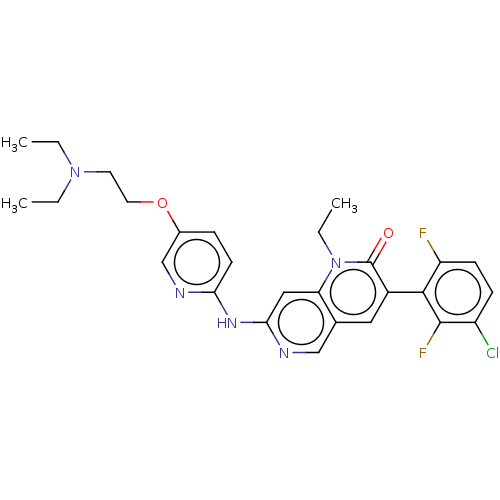 (US9714247, 97) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM264378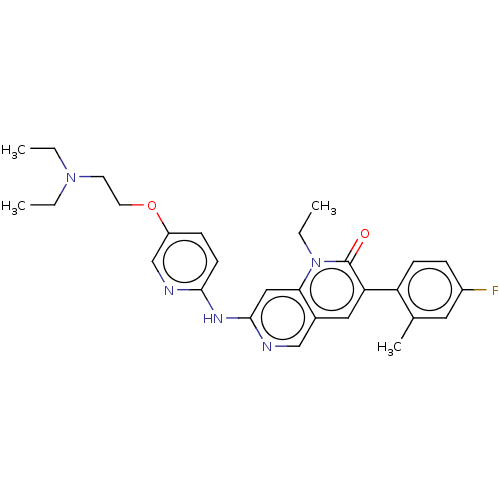 (US9714247, 99) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM264380 (US9714247, 101) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM264384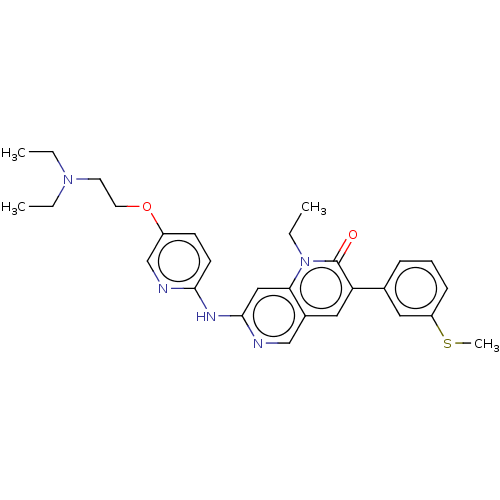 (US9714247, 105) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264398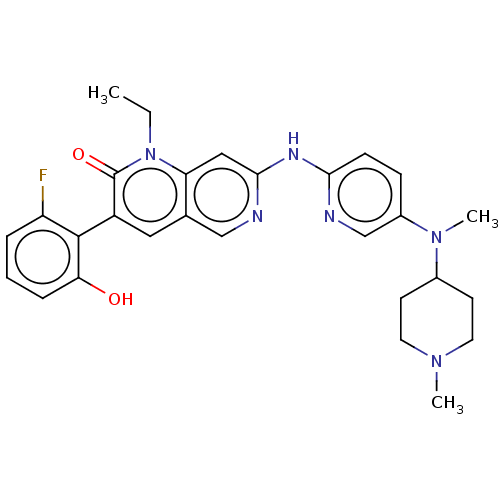 (US9714247, 120) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM264409 (US9714247, 131) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264413 (US9714247, 135) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM264423 (US9714247, 145 | US9714247, 146) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM264421 (US9714247, 143 | US9714247, 144) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM264421 (US9714247, 143 | US9714247, 144) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM264421 (US9714247, 143 | US9714247, 144) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM264423 (US9714247, 145 | US9714247, 146) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM264425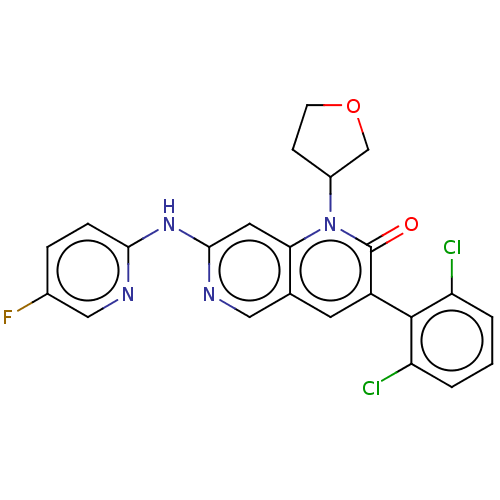 (US9714247, 147 | US9714247, 148) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tolero Pharmaceuticals, Inc.; Mannkind Corporation US Patent | Assay Description A concentration of a drug that reduces the observed activity of an enzyme by 50%. An IC50 is not a true affinity constant and the specific value dete... | US Patent US9714247 (2017) BindingDB Entry DOI: 10.7270/Q2BR8V6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 452 total ) | Next | Last >> |