| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Adenosine receptor A2a |
|---|
| Ligand | BDBM551480 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Measurement of A2a Binding Affinity Using Radioligand Binding |
|---|
| IC50 | 0.600±n/a nM |
|---|
| Citation |  Larsen, MA; Ali, A; Cumming, J; DeMong, D; Deng, Q; Graham, TH; Hennessy, E; Hoover, AJ; Liu, P; Liu, K; Mansoor, UF; Pan, J; Plummer, CW; Sather, A; Swaminathan, U; Wang, H; Zhang, Y 9-substituted amino triazolo quinazoline derivatives as adenosine receptor antagonists, pharmaceutical compositions and their use US Patent US11312719 Publication Date 4/26/2022 Larsen, MA; Ali, A; Cumming, J; DeMong, D; Deng, Q; Graham, TH; Hennessy, E; Hoover, AJ; Liu, P; Liu, K; Mansoor, UF; Pan, J; Plummer, CW; Sather, A; Swaminathan, U; Wang, H; Zhang, Y 9-substituted amino triazolo quinazoline derivatives as adenosine receptor antagonists, pharmaceutical compositions and their use US Patent US11312719 Publication Date 4/26/2022 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Adenosine receptor A2a |
|---|
| Name: | Adenosine receptor A2a |
|---|
| Synonyms: | A2A adenosine receptor (hA2A) | AA2AR_HUMAN | ADENOSINE A2 | ADENOSINE A2a | ADORA2 | ADORA2A | Adenosine A2A receptor (A2AAR) |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 44716.46 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P29274 |
|---|
| Residue: | 412 |
|---|
| Sequence: | MPIMGSSVYITVELAIAVLAILGNVLVCWAVWLNSNLQNVTNYFVVSLAAADIAVGVLAI
PFAITISTGFCAACHGCLFIACFVLVLTQSSIFSLLAIAIDRYIAIRIPLRYNGLVTGTR
AKGIIAICWVLSFAIGLTPMLGWNNCGQPKEGKNHSQGCGEGQVACLFEDVVPMNYMVYF
NFFACVLVPLLLMLGVYLRIFLAARRQLKQMESQPLPGERARSTLQKEVHAAKSLAIIVG
LFALCWLPLHIINCFTFFCPDCSHAPLWLMYLAIVLSHTNSVVNPFIYAYRIREFRQTFR
KIIRSHVLRQQEPFKAAGTSARVLAAHGSDGEQVSLRLNGHPPGVWANGSAPHPERRPNG
YALGLVSGGSAQESQGNTGLPDVELLSHELKGVCPEPPGLDDPLAQDGAGVS
|
|
|
|---|
| BDBM551480 |
|---|
| n/a |
|---|
| Name | BDBM551480 |
|---|
| Synonyms: | (R)-5-(3-(5-amino-9-fluoro-8-methoxy-[1,2,4]triazolo[1,5-c]quinazolin- 2-yl)piperidin-1-yl)-1-(difluoromethyl)-3-methylpyridin-2(1H)-one | US11312719, Example 25 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H22F3N7O2 |
|---|
| Mol. Mass. | 473.451 |
|---|
| SMILES | COc1cc2nc(N)n3nc(nc3c2cc1F)[C@@H]1CCCN(C1)c1cc(C)c(=O)n(c1)C(F)F |r| |
|---|
| Structure | 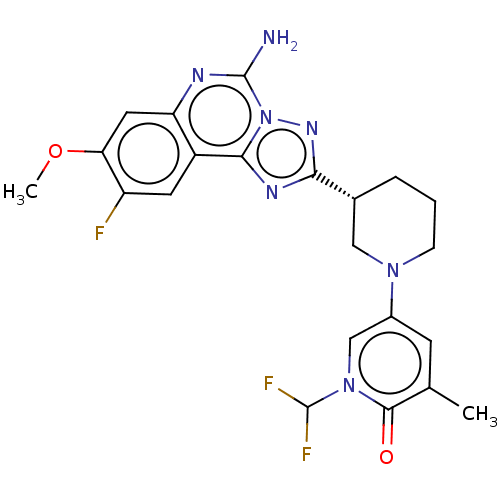
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Larsen, MA; Ali, A; Cumming, J; DeMong, D; Deng, Q; Graham, TH; Hennessy, E; Hoover, AJ; Liu, P; Liu, K; Mansoor, UF; Pan, J; Plummer, CW; Sather, A; Swaminathan, U; Wang, H; Zhang, Y 9-substituted amino triazolo quinazoline derivatives as adenosine receptor antagonists, pharmaceutical compositions and their use US Patent US11312719 Publication Date 4/26/2022
Larsen, MA; Ali, A; Cumming, J; DeMong, D; Deng, Q; Graham, TH; Hennessy, E; Hoover, AJ; Liu, P; Liu, K; Mansoor, UF; Pan, J; Plummer, CW; Sather, A; Swaminathan, U; Wang, H; Zhang, Y 9-substituted amino triazolo quinazoline derivatives as adenosine receptor antagonists, pharmaceutical compositions and their use US Patent US11312719 Publication Date 4/26/2022