 Found 1420 hits with Last Name = 'graham' and Initial = 'th'
Found 1420 hits with Last Name = 'graham' and Initial = 'th' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50443348
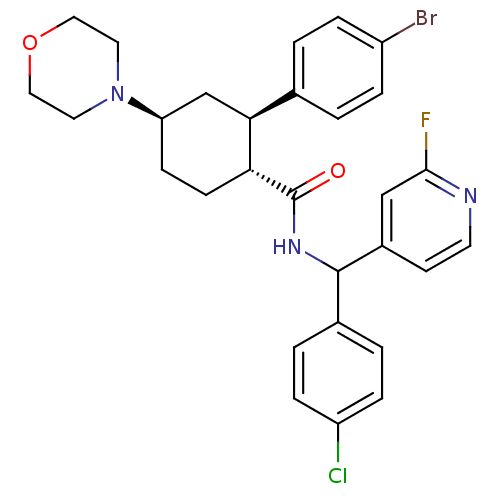
(CHEMBL3086040 | US8669252, 12)Show SMILES Fc1cc(ccn1)C(NC(=O)[C@@H]1CC[C@H](C[C@H]1c1ccc(Br)cc1)N1CCOCC1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C29H30BrClFN3O2/c30-22-5-1-19(2-6-22)26-18-24(35-13-15-37-16-14-35)9-10-25(26)29(36)34-28(20-3-7-23(31)8-4-20)21-11-12-33-27(32)17-21/h1-8,11-12,17,24-26,28H,9-10,13-16,18H2,(H,34,36)/t24-,25-,26+,28?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem Lett 23: 6228-33 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.094
BindingDB Entry DOI: 10.7270/Q2DZ09Q4 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM551542

(US11312719, Example 88)Show SMILES COc1cc2nc(N)n3nc(nc3c2cc1F)[C@@H]1CC[C@H](C)N(C1)c1cnn(c1)C1CCCC1O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639SZ0 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50443348

(CHEMBL3086040 | US8669252, 12)Show SMILES Fc1cc(ccn1)C(NC(=O)[C@@H]1CC[C@H](C[C@H]1c1ccc(Br)cc1)N1CCOCC1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C29H30BrClFN3O2/c30-22-5-1-19(2-6-22)26-18-24(35-13-15-37-16-14-35)9-10-25(26)29(36)34-28(20-3-7-23(31)8-4-20)21-11-12-33-27(32)17-21/h1-8,11-12,17,24-26,28H,9-10,13-16,18H2,(H,34,36)/t24-,25-,26+,28?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The potency of compounds of formula I against PrCP was determined by a fluorescence intensity kinetic assay measuring the IC50 values of PrCP inhibit... |
US Patent US8669252 (2014)
BindingDB Entry DOI: 10.7270/Q2J9652X |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50443351
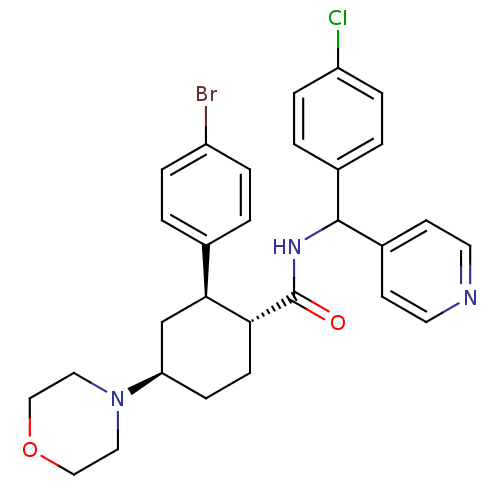
(CHEMBL3086037 | US8669252, 15)Show SMILES Clc1ccc(cc1)C(NC(=O)[C@@H]1CC[C@H](C[C@H]1c1ccc(Br)cc1)N1CCOCC1)c1ccncc1 |r| Show InChI InChI=1S/C29H31BrClN3O2/c30-23-5-1-20(2-6-23)27-19-25(34-15-17-36-18-16-34)9-10-26(27)29(35)33-28(22-11-13-32-14-12-22)21-3-7-24(31)8-4-21/h1-8,11-14,25-28H,9-10,15-19H2,(H,33,35)/t25-,26-,27+,28?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The potency of compounds of formula I against PrCP was determined by a fluorescence intensity kinetic assay measuring the IC50 values of PrCP inhibit... |
US Patent US8669252 (2014)
BindingDB Entry DOI: 10.7270/Q2J9652X |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM119411
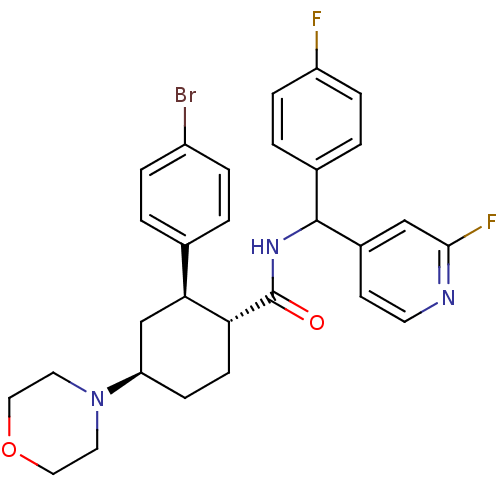
(US8669252, 43)Show SMILES Fc1ccc(cc1)C(NC(=O)[C@@H]1CC[C@H](C[C@H]1c1ccc(Br)cc1)N1CCOCC1)c1ccnc(F)c1 |r| Show InChI InChI=1S/C29H30BrF2N3O2/c30-22-5-1-19(2-6-22)26-18-24(35-13-15-37-16-14-35)9-10-25(26)29(36)34-28(20-3-7-23(31)8-4-20)21-11-12-33-27(32)17-21/h1-8,11-12,17,24-26,28H,9-10,13-16,18H2,(H,34,36)/t24-,25-,26+,28?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The potency of compounds of formula I against PrCP was determined by a fluorescence intensity kinetic assay measuring the IC50 values of PrCP inhibit... |
US Patent US8669252 (2014)
BindingDB Entry DOI: 10.7270/Q2J9652X |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50443351

(CHEMBL3086037 | US8669252, 15)Show SMILES Clc1ccc(cc1)C(NC(=O)[C@@H]1CC[C@H](C[C@H]1c1ccc(Br)cc1)N1CCOCC1)c1ccncc1 |r| Show InChI InChI=1S/C29H31BrClN3O2/c30-23-5-1-20(2-6-23)27-19-25(34-15-17-36-18-16-34)9-10-26(27)29(35)33-28(22-11-13-32-14-12-22)21-3-7-24(31)8-4-21/h1-8,11-14,25-28H,9-10,15-19H2,(H,33,35)/t25-,26-,27+,28?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem Lett 23: 6228-33 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.094
BindingDB Entry DOI: 10.7270/Q2DZ09Q4 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382899

(CHEMBL2022787)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(C)c(Cl)c1 |r| Show InChI InChI=1S/C31H37ClF2N4O/c1-19-6-8-23(16-27(19)32)38-29(14-20(2)35-38)21-10-12-36(13-11-21)30(39)26-18-37(31(3,4)5)17-25(26)24-9-7-22(33)15-28(24)34/h6-9,14-16,21,25-26H,10-13,17-18H2,1-5H3/t25-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM551571

(US11312719, Example 117)Show SMILES COc1cc(F)cc2c3nc(nn3c(N)nc12)[C@@H]1CC[C@H](C)N(C1)c1cn(CC(C)(C)O)nc1C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639SZ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM551564

(1-(4-((2S,5R)-5-(5-amino-8-chloro-9-fluoro-[1,2,4]...)Show SMILES C[C@H]1CC[C@H](CN1c1cnn(CC(C)(C)O)c1)c1nc2c3cc(F)c(Cl)cc3nc(N)n2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639SZ0 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50383421
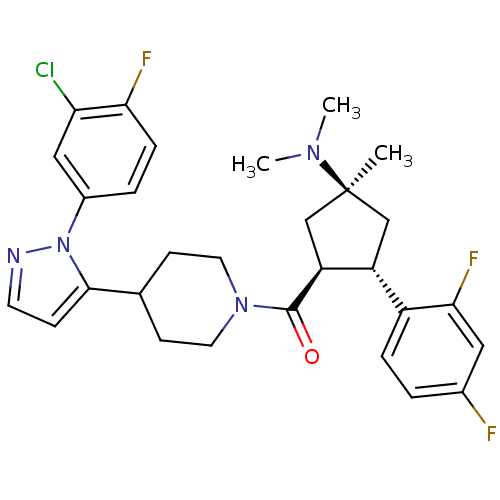
(CHEMBL2031595)Show SMILES CN(C)[C@]1(C)C[C@@H]([C@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1ccnn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C29H32ClF3N4O/c1-29(35(2)3)16-22(21-6-4-19(31)14-26(21)33)23(17-29)28(38)36-12-9-18(10-13-36)27-8-11-34-37(27)20-5-7-25(32)24(30)15-20/h4-8,11,14-15,18,22-23H,9-10,12-13,16-17H2,1-3H3/t22-,23+,29+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM551543
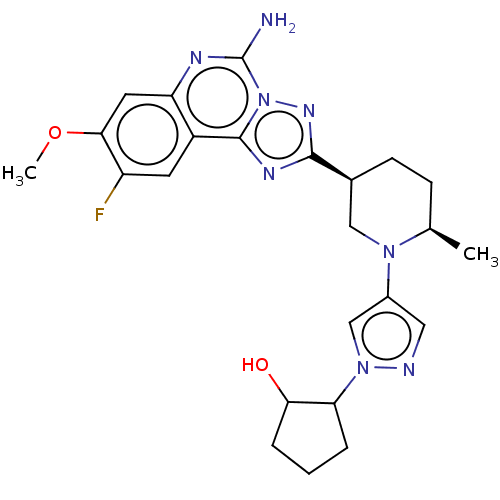
(US11312719, Example 89)Show SMILES COc1cc2nc(N)n3nc(nc3c2cc1F)[C@H]1CC[C@@H](C)N(C1)c1cnn(c1)C1CCCC1O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639SZ0 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382913
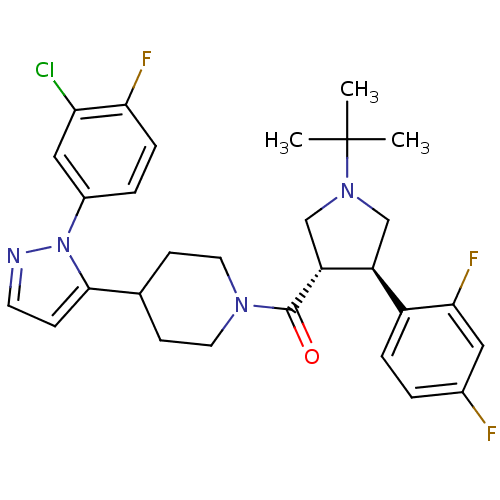
(CHEMBL2023210)Show SMILES CC(C)(C)N1C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1ccnn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C29H32ClF3N4O/c1-29(2,3)36-16-22(21-6-4-19(31)14-26(21)33)23(17-36)28(38)35-12-9-18(10-13-35)27-8-11-34-37(27)20-5-7-25(32)24(30)15-20/h4-8,11,14-15,18,22-23H,9-10,12-13,16-17H2,1-3H3/t22-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382904
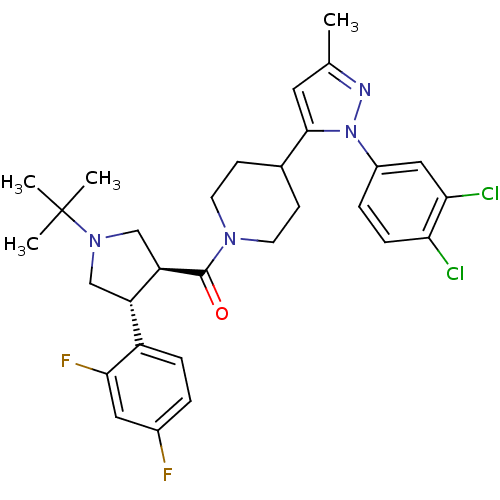
(CHEMBL2022793)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-28(38(35-18)21-6-8-25(31)26(32)15-21)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-5-20(33)14-27(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50443348

(CHEMBL3086040 | US8669252, 12)Show SMILES Fc1cc(ccn1)C(NC(=O)[C@@H]1CC[C@H](C[C@H]1c1ccc(Br)cc1)N1CCOCC1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C29H30BrClFN3O2/c30-22-5-1-19(2-6-22)26-18-24(35-13-15-37-16-14-35)9-10-25(26)29(36)34-28(20-3-7-23(31)8-4-20)21-11-12-33-27(32)17-21/h1-8,11-12,17,24-26,28H,9-10,13-16,18H2,(H,34,36)/t24-,25-,26+,28?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mouse PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem Lett 23: 6228-33 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.094
BindingDB Entry DOI: 10.7270/Q2DZ09Q4 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM551504
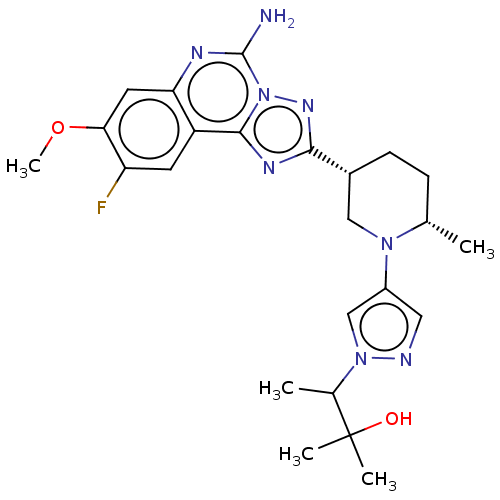
(US11312719, Example 49)Show SMILES COc1cc2nc(N)n3nc(nc3c2cc1F)[C@@H]1CC[C@H](C)N(C1)c1cnn(c1)C(C)C(C)(C)O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639SZ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM551476

((R)-1-(4-(3-(5-amino-9-fluoro-8-methoxy-[1,2,4]tri...)Show SMILES COc1cc2nc(N)n3nc(nc3c2cc1F)[C@@H]1CCCN(C1)c1cn(CC(C)(C)O)nc1C1CC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639SZ0 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609742
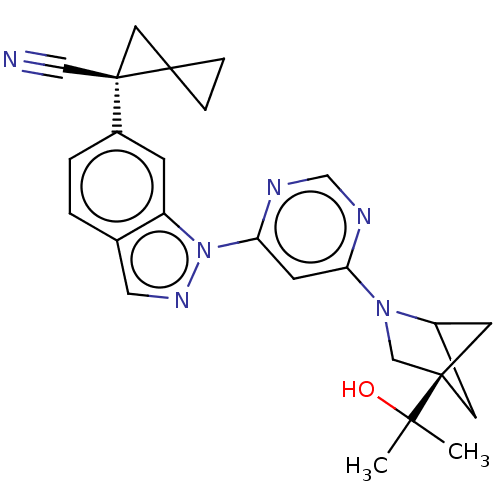
(CHEMBL5286106) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609742

(CHEMBL5286106) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609728

(CHEMBL5267350) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM551576
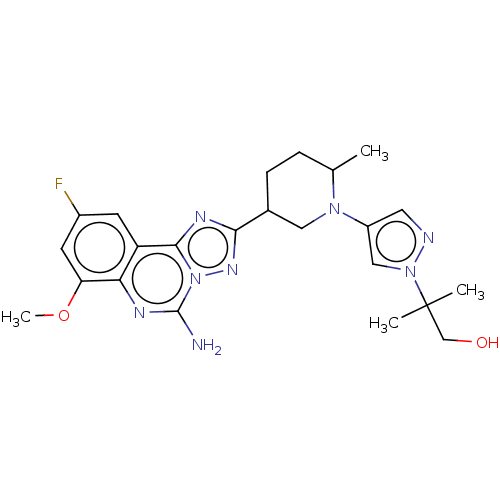
(2-(4-((2R or 2S,5R or 5S)-5-(5-amino-9-fluoro-7-me...)Show SMILES COc1cc(F)cc2c3nc(nn3c(N)nc12)C1CCC(C)N(C1)c1cnn(c1)C(C)(C)CO | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639SZ0 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382906

(CHEMBL2023202)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-27(38(35-18)28-14-20(31)5-8-25(28)32)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50382913

(CHEMBL2023210)Show SMILES CC(C)(C)N1C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1ccnn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C29H32ClF3N4O/c1-29(2,3)36-16-22(21-6-4-19(31)14-26(21)33)23(17-36)28(38)35-12-9-18(10-13-35)27-8-11-34-37(27)20-5-7-25(32)24(30)15-20/h4-8,11,14-15,18,22-23H,9-10,12-13,16-17H2,1-3H3/t22-,23+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382906

(CHEMBL2023202)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-27(38(35-18)28-14-20(31)5-8-25(28)32)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50361786
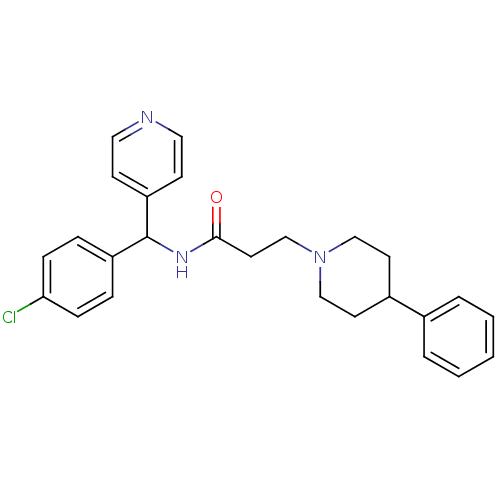
(CHEMBL1938522)Show SMILES Clc1ccc(cc1)C(NC(=O)CCN1CCC(CC1)c1ccccc1)c1ccncc1 Show InChI InChI=1S/C26H28ClN3O/c27-24-8-6-22(7-9-24)26(23-10-15-28-16-11-23)29-25(31)14-19-30-17-12-21(13-18-30)20-4-2-1-3-5-20/h1-11,15-16,21,26H,12-14,17-19H2,(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by continuous fluorometric assay |
Bioorg Med Chem Lett 22: 658-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.060
BindingDB Entry DOI: 10.7270/Q2B56K50 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM551580

(US11312719, Example 126 | US11312719, Example 127 ...)Show SMILES COc1cc2nc(N)n3nc(nc3c2cc1F)C1CCC(C)N(C1)c1cnn(c1)C(C)(C)CO | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639SZ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM551576

(2-(4-((2R or 2S,5R or 5S)-5-(5-amino-9-fluoro-7-me...)Show SMILES COc1cc(F)cc2c3nc(nn3c(N)nc12)C1CCC(C)N(C1)c1cnn(c1)C(C)(C)CO | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639SZ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM551540
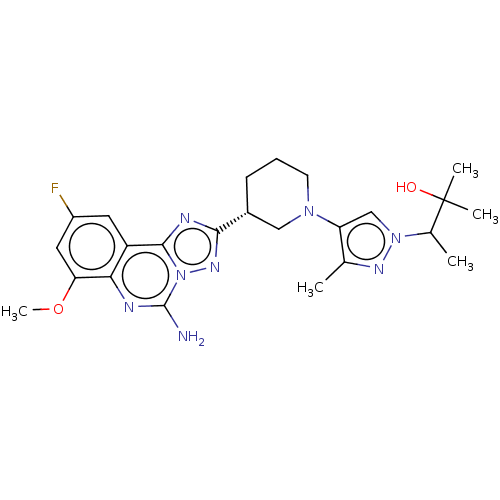
((S or R)-3-(4-((R)-3-(5-amino-9-fluoro-7-methoxy-[...)Show SMILES COc1cc(F)cc2c3nc(nn3c(N)nc12)[C@@H]1CCCN(C1)c1cn(nc1C)C(C)C(C)(C)O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639SZ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM551485
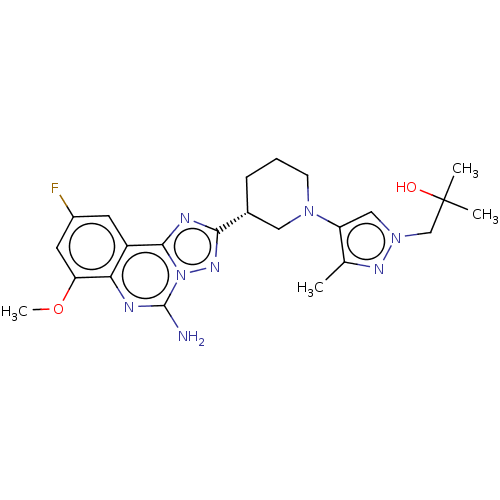
((R)-1-(4-(3-(5-amino-9-fluoro-7-methoxy-[1,2,4]tri...)Show SMILES COc1cc(F)cc2c3nc(nn3c(N)nc12)[C@@H]1CCCN(C1)c1cn(CC(C)(C)O)nc1C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639SZ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM551678
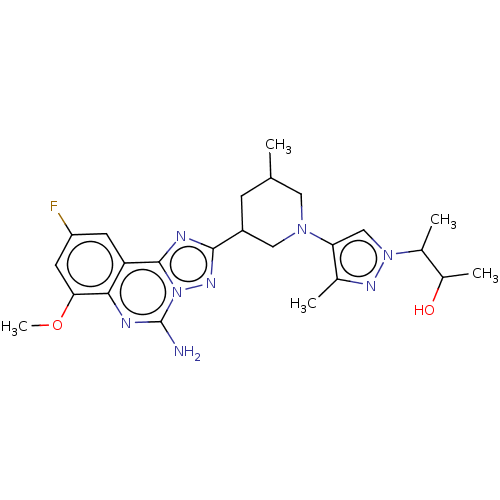
((2S,3S or 2R,3R)-3-(4-((3R,5S or 3S,5R)-3-(5-amino...)Show SMILES COc1cc(F)cc2c3nc(nn3c(N)nc12)C1CC(C)CN(C1)c1cn(nc1C)C(C)C(C)O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The ability of compounds to antagonize human A2A and A2B adenosine receptors was determined using a kit to measure changes in intracellular cyclic AM... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639SZ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM551666
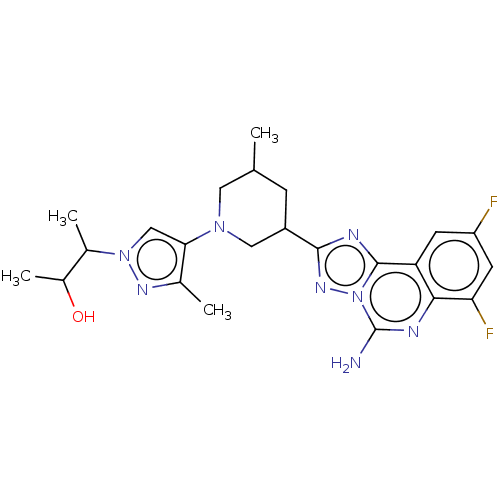
((2S,3S or 2R,3R)-3-(4-((3R,5S or 3S,5R)-3-(5-amino...)Show SMILES CC(O)C(C)n1cc(N2CC(C)CC(C2)c2nc3c4cc(F)cc(F)c4nc(N)n3n2)c(C)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The ability of compounds to antagonize human A2A and A2B adenosine receptors was determined using a kit to measure changes in intracellular cyclic AM... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639SZ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM551678

((2S,3S or 2R,3R)-3-(4-((3R,5S or 3S,5R)-3-(5-amino...)Show SMILES COc1cc(F)cc2c3nc(nn3c(N)nc12)C1CC(C)CN(C1)c1cn(nc1C)C(C)C(C)O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The ability of compounds to antagonize human A2A and A2B adenosine receptors was determined using a kit to measure changes in intracellular cyclic AM... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639SZ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM551540

((S or R)-3-(4-((R)-3-(5-amino-9-fluoro-7-methoxy-[...)Show SMILES COc1cc(F)cc2c3nc(nn3c(N)nc12)[C@@H]1CCCN(C1)c1cn(nc1C)C(C)C(C)(C)O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639SZ0 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50382906

(CHEMBL2023202)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-27(38(35-18)28-14-20(31)5-8-25(28)32)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382905

(CHEMBL2022794)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-28(38(35-18)27-8-5-20(31)14-25(27)32)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM551524
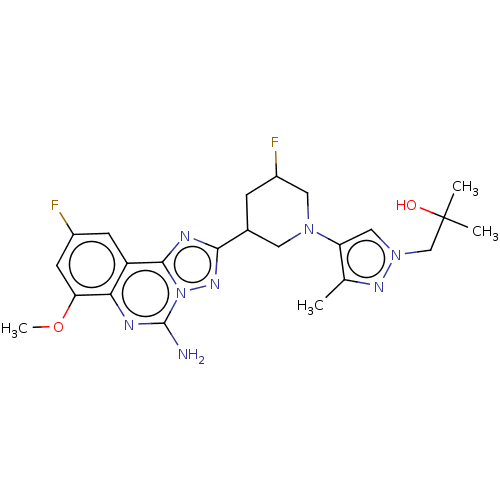
(1-(4-((3R,5S or 3S,5R)-3-(5-amino-9-fluoro-7-metho...)Show SMILES COc1cc(F)cc2c3nc(nn3c(N)nc12)C1CC(F)CN(C1)c1cn(CC(C)(C)O)nc1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639SZ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM551566

(1-(4-((2S,5R)-5-(5-amino-9-fluoro-8-methyl-[1,2,4]...)Show SMILES C[C@H]1CC[C@H](CN1c1cnn(CC(C)(C)O)c1)c1nc2c3cc(F)c(C)cc3nc(N)n2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639SZ0 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50383421

(CHEMBL2031595)Show SMILES CN(C)[C@]1(C)C[C@@H]([C@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1ccnn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C29H32ClF3N4O/c1-29(35(2)3)16-22(21-6-4-19(31)14-26(21)33)23(17-29)28(38)36-12-9-18(10-13-36)27-8-11-34-37(27)20-5-7-25(32)24(30)15-20/h4-8,11,14-15,18,22-23H,9-10,12-13,16-17H2,1-3H3/t22-,23+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50361774
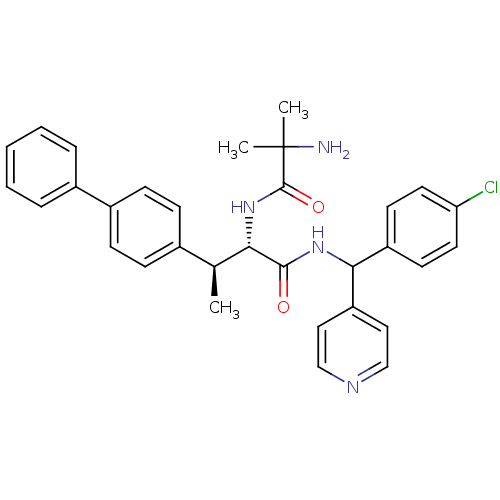
(CHEMBL1938510)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)NC(c1ccncc1)c1ccc(Cl)cc1)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C32H33ClN4O2/c1-21(22-9-11-24(12-10-22)23-7-5-4-6-8-23)28(37-31(39)32(2,3)34)30(38)36-29(26-17-19-35-20-18-26)25-13-15-27(33)16-14-25/h4-21,28-29H,34H2,1-3H3,(H,36,38)(H,37,39)/t21-,28-,29?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mouse PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by continuous fluorometric assay |
Bioorg Med Chem Lett 22: 658-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.060
BindingDB Entry DOI: 10.7270/Q2B56K50 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609741

(CHEMBL5284341) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50383427
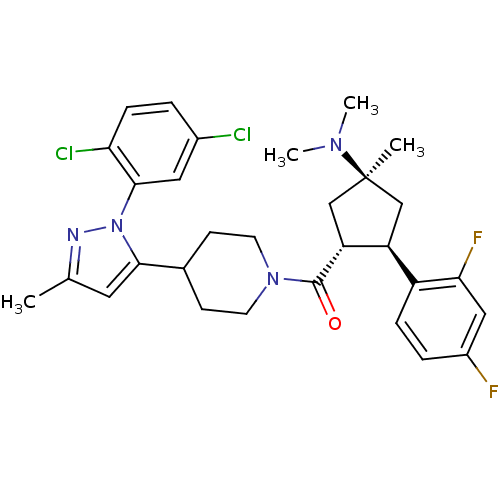
(CHEMBL2031588)Show SMILES CN(C)[C@]1(C)C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1cc(C)nn1-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-27(38(35-18)28-14-20(31)5-8-25(28)32)19-9-11-37(12-10-19)29(39)24-17-30(2,36(3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+,30-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50382906

(CHEMBL2023202)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-27(38(35-18)28-14-20(31)5-8-25(28)32)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50383425
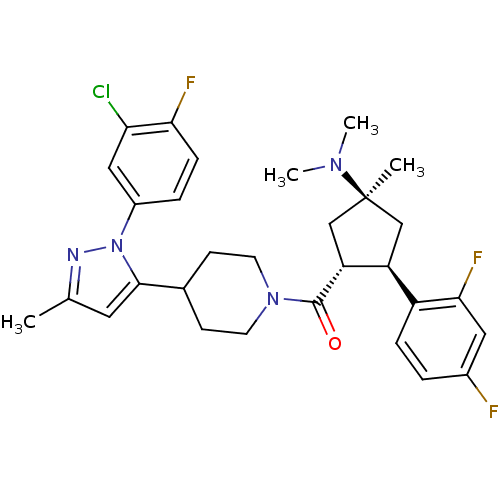
(CHEMBL2031590)Show SMILES CN(C)[C@]1(C)C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1cc(C)nn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C30H34ClF3N4O/c1-18-13-28(38(35-18)21-6-8-26(33)25(31)15-21)19-9-11-37(12-10-19)29(39)24-17-30(2,36(3)4)16-23(24)22-7-5-20(32)14-27(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+,30-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382912

(CHEMBL2023209 | US8569299, 4)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C30H34ClF3N4O/c1-18-13-28(38(35-18)21-6-8-26(33)25(31)15-21)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-5-20(32)14-27(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382910
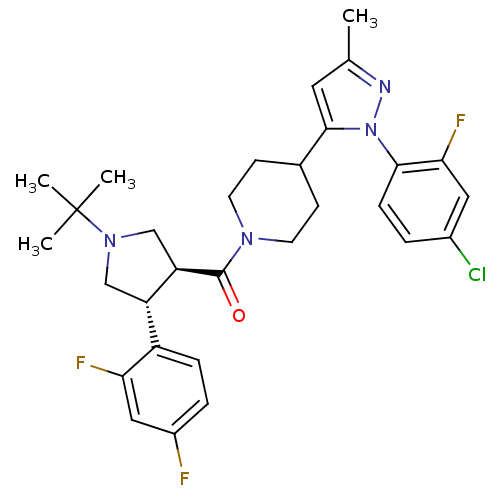
(CHEMBL2023207)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(Cl)cc1F |r| Show InChI InChI=1S/C30H34ClF3N4O/c1-18-13-28(38(35-18)27-8-5-20(31)14-26(27)34)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(32)15-25(22)33/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM257207
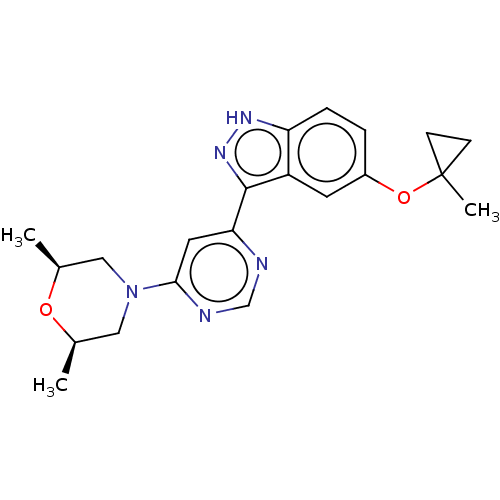
(US9493440, 51)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1cc(ncn1)-c1n[nH]c2ccc(OC3(C)CC3)cc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382912

(CHEMBL2023209 | US8569299, 4)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C30H34ClF3N4O/c1-18-13-28(38(35-18)21-6-8-26(33)25(31)15-21)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-5-20(32)14-27(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Merck Sharp & Dohme Corp
US Patent
| Assay Description
The potency of compounds against PRCP was determined by a fluorescence intensity kinetic assay measuring the IC50 values of PRCP inhibitor test compo... |
US Patent US8569299 (2013)
BindingDB Entry DOI: 10.7270/Q2K9365V |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM551643
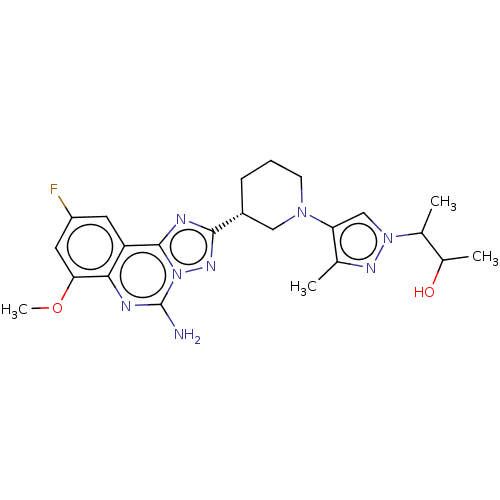
((2S,3S or 2R,3R)-3-(4-((R)-3-(5-amino-9-fluoro-7- ...)Show SMILES COc1cc(F)cc2c3nc(nn3c(N)nc12)[C@@H]1CCCN(C1)c1cn(nc1C)C(C)C(C)O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The ability of compounds to antagonize human A2A and A2B adenosine receptors was determined using a kit to measure changes in intracellular cyclic AM... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639SZ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM551666

((2S,3S or 2R,3R)-3-(4-((3R,5S or 3S,5R)-3-(5-amino...)Show SMILES CC(O)C(C)n1cc(N2CC(C)CC(C2)c2nc3c4cc(F)cc(F)c4nc(N)n3n2)c(C)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The ability of compounds to antagonize human A2A and A2B adenosine receptors was determined using a kit to measure changes in intracellular cyclic AM... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639SZ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM551464
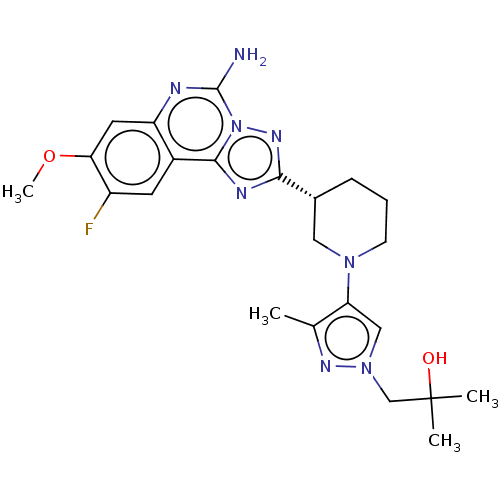
((R)-1-(4-(3-(5-amino-9-fluoro-8-methoxy-[1,2,4]tri...)Show SMILES COc1cc2nc(N)n3nc(nc3c2cc1F)[C@@H]1CCCN(C1)c1cn(CC(C)(C)O)nc1C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639SZ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM551674
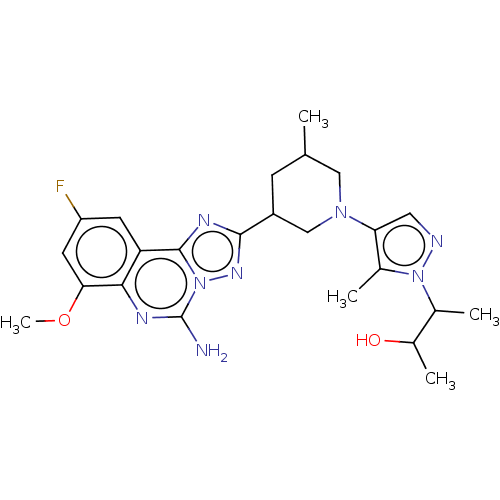
((2S,3S or 2R,3R)-3-(4-((3R,5S or 3S,5R)-3-(5-amino...)Show SMILES COc1cc(F)cc2c3nc(nn3c(N)nc12)C1CC(C)CN(C1)c1cnn(C(C)C(C)O)c1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The ability of compounds to antagonize human A2A and A2B adenosine receptors was determined using a kit to measure changes in intracellular cyclic AM... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639SZ0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data