

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | GTPase KRas [G12C] | ||
| Ligand | BDBM609011 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | Scintillation Proximity Assay (SPA) | ||
| IC50 | 8.00±n/a nM | ||
| Citation |  Cotesta, S; Gerspacher, M; Leblanc, C; Lorthiois, EL; Liu, B; Machauer, R; Mah, R; Mura, C; Rigollier, P; Schneider, N; Stutz, S; Vaupel, A; Warin, N; Wilcken, R Pyrazolyl derivatives useful as anti-cancer agents US Patent US11702409 Publication Date 7/18/2023 Cotesta, S; Gerspacher, M; Leblanc, C; Lorthiois, EL; Liu, B; Machauer, R; Mah, R; Mura, C; Rigollier, P; Schneider, N; Stutz, S; Vaupel, A; Warin, N; Wilcken, R Pyrazolyl derivatives useful as anti-cancer agents US Patent US11702409 Publication Date 7/18/2023 | ||
| More Info.: | Get all data from this article, Assay Method | ||
| GTPase KRas [G12C] | |||
| Name: | GTPase KRas [G12C] | ||
| Synonyms: | GTPase KRas (G12C) | KRAS | KRAS2 | RASK2 | RASK_HUMAN | ||
| Type: | Enzyme Catalytic Domain | ||
| Mol. Mass.: | 21702.19 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P01116[G12C] | ||
| Residue: | 189 | ||
| Sequence: |
| ||
| BDBM609011 | |||
| n/a | |||
| Name | BDBM609011 | ||
| Synonyms: | US11702409, Example 80a | US11702409, Example 80b | ||
| Type | Small organic molecule | ||
| Emp. Form. | C38H46ClN7O6S | ||
| Mol. Mass. | 764.333 | ||
| SMILES | Cc1c(c(nn1C1CC2(C1)CN(C2)C(=O)OC(C)(C)C)-c1ccc2nn(CCOS(C)(=O)=O)cc2c1)-c1c(Cl)c(C)cc2n(ncc12)C1CCCCO1 |(-8.56,21.58,;-7.07,21.98,;-5.82,21.07,;-4.58,21.98,;-5.05,23.44,;-6.59,23.44,;-7.36,24.77,;-6.96,26.26,;-8.45,26.66,;-8.85,25.17,;-8.05,28.15,;-9.54,28.55,;-9.94,27.06,;-10.63,29.64,;-10.23,31.12,;-12.12,29.24,;-13.21,30.33,;-14.69,29.93,;-12.81,31.81,;-11.72,30.72,;-3.09,21.58,;-2,22.67,;-.51,22.27,;-.11,20.78,;1.26,20.08,;1.02,18.56,;2.11,17.47,;1.71,15.98,;2.8,14.89,;2.4,13.41,;3.89,13.01,;2,11.92,;.91,13.81,;-.5,18.32,;-1.2,19.69,;-2.69,20.09,;-5.82,19.53,;-7.16,18.76,;-8.49,19.53,;-7.16,17.22,;-8.49,16.45,;-5.82,16.45,;-4.49,17.22,;-3.02,16.74,;-2.12,17.99,;-3.02,19.24,;-4.49,18.76,;-2.55,15.28,;-3.58,14.14,;-3.1,12.67,;-1.6,12.35,;-.57,13.5,;-1.04,14.96,)| | ||
| Structure | 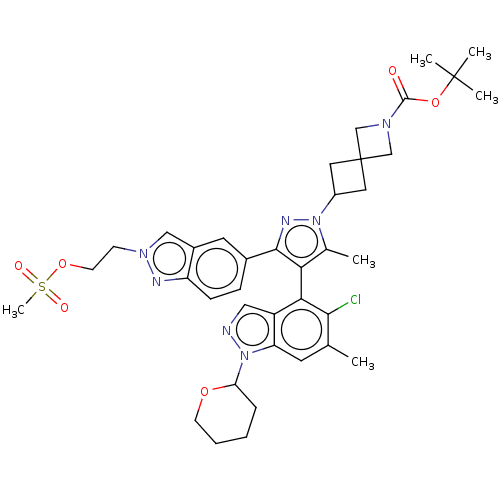
| ||