

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM18313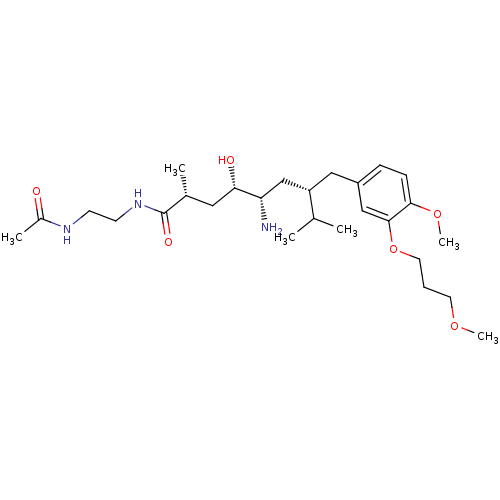 ((2R,4S,5S,7S)-5-amino-N-(2-acetamidoethyl)-4-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110357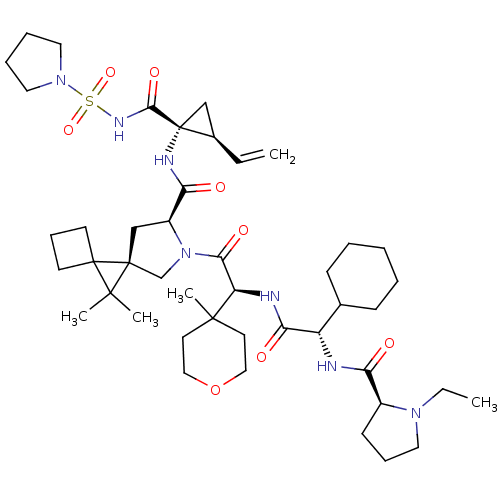 (US8613914, 125 | US9206232, 129) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM196124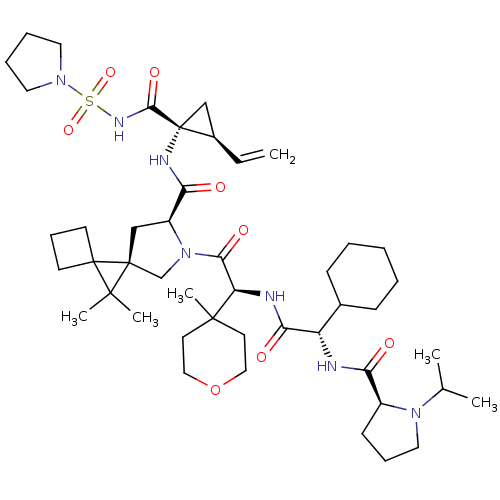 (US9206232, 125) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18288 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18342 ((2S,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18337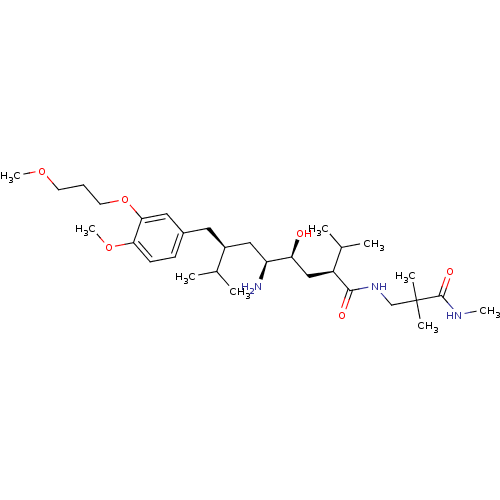 ((2S,4S,5S,7S)-5-amino-N-[2,2-dimethyl-2-(methylcar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110396 (US8613914, 170 | US9206232, 170) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110396 (US8613914, 170 | US9206232, 170) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18278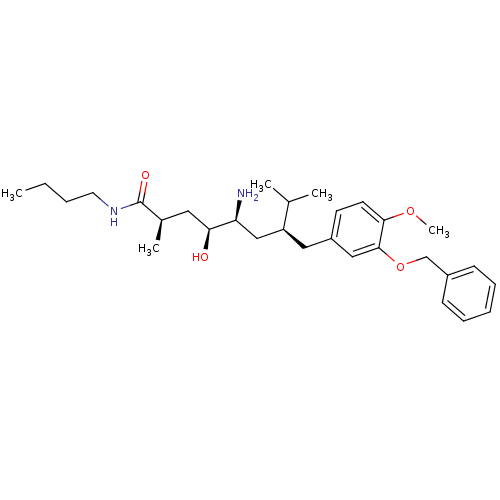 ((2R,4S,5S,7S)-5-amino-7-{[3-(benzyloxy)-4-methoxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18340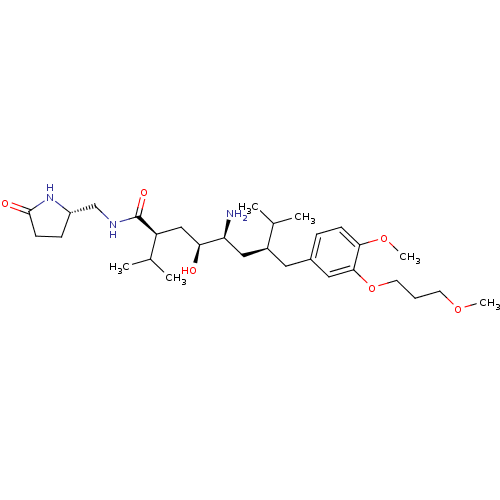 ((2S,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110393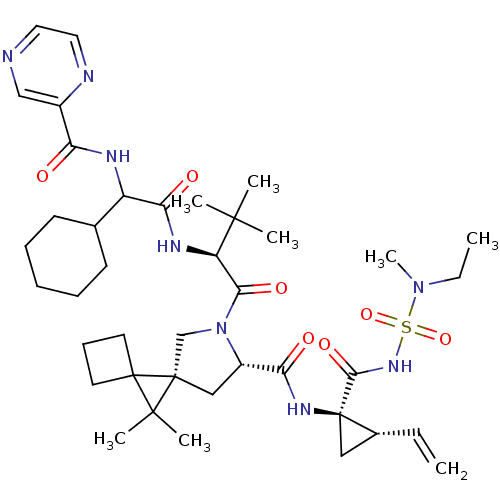 (US8613914, 167 | US9206232, 167) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110400 (US8613914, 174 | US9206232, 174) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18344 ((2S,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110400 (US8613914, 174 | US9206232, 174) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110393 (US8613914, 167 | US9206232, 167) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17950 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089055 (CHEMBL273811 | N*2*-(4-Ethanesulfonylmethyl-phenyl...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18323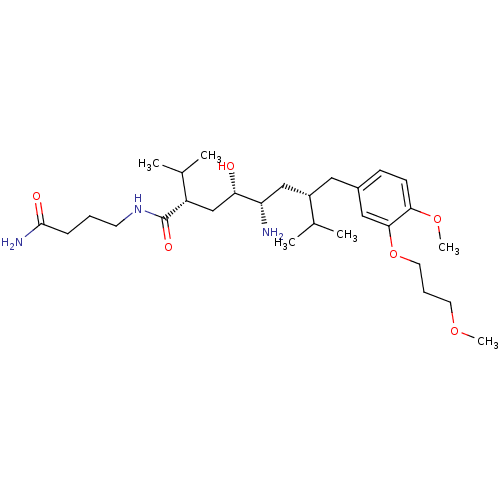 ((2S,4S,5S,7S)-5-amino-N-(3-carbamoylpropyl)-4-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18338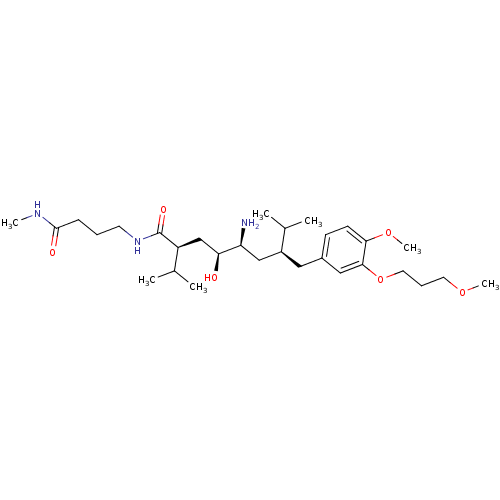 ((2S,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110388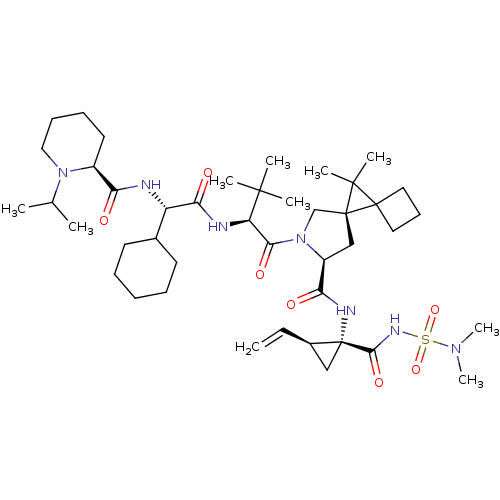 (US8613914, 162 | US9206232, 162) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18326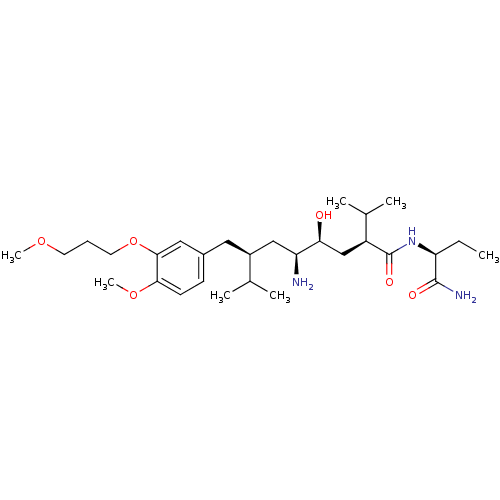 ((2S,4S,5S,7S)-5-amino-N-[(1S)-1-carbamoylpropyl]-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18289 ((2R,4S,5S,7S)-5-amino-4-hydroxy-N-(4-hydroxybutyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089048 (CHEMBL18163 | N,N-Dimethyl-C-[4-(4-phenylamino-qui...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110388 (US8613914, 162 | US9206232, 162) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM196120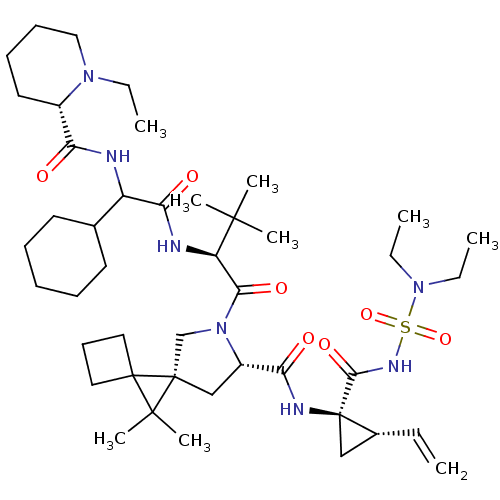 (US9206232, 77) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110280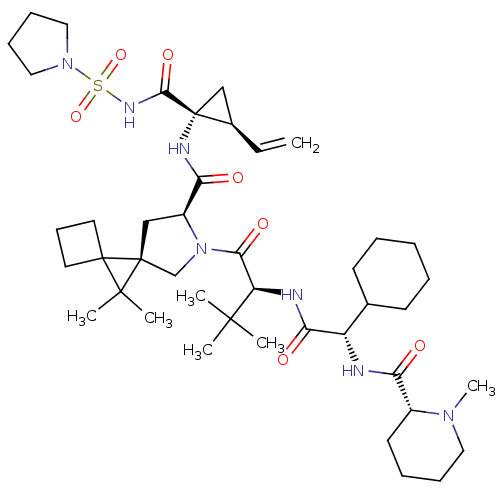 (US8613914, 48 | US9206232, 48) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110273 (US8613914, 41 | US9206232, 41) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110388 (US8613914, 162 | US9206232, 162) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110280 (US8613914, 48 | US9206232, 48) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110273 (US8613914, 41 | US9206232, 41) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18336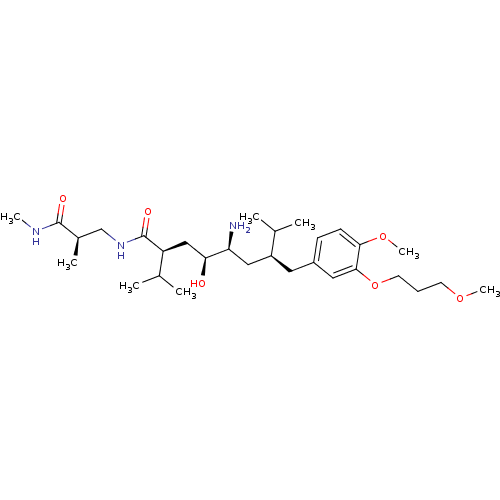 ((2S,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18334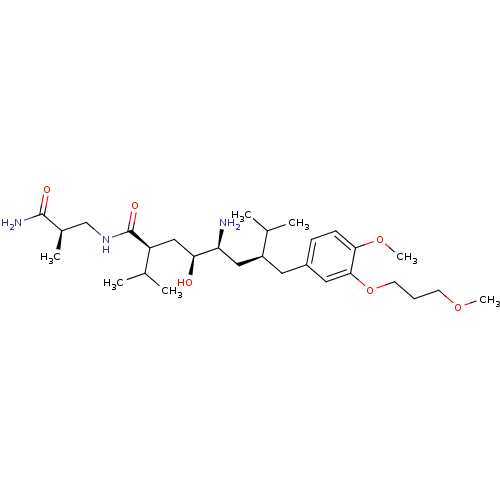 ((2S,4S,5S,7S)-5-amino-N-[(2R)-2-carbamoyl-2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110309 (US8613914, 77) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18308 ((2R,4S,5S,7S)-5-amino-N-(3-carbamoylpropyl)-4-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110383 (US8613914, 157 | US9206232, 157) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18304 (8-phenyl-octanecarboxamide peptidomimetic, 61 | me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17949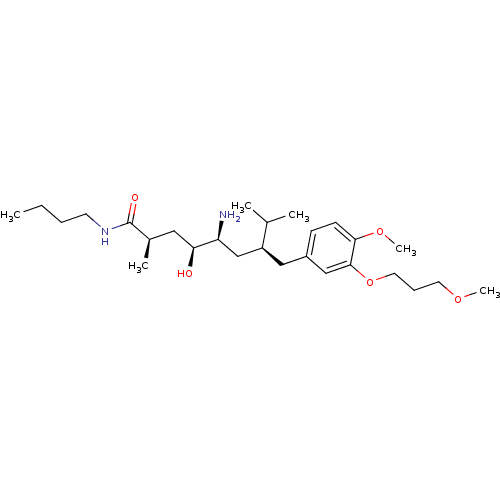 ((2R,4S,5S,7S)-5-amino-N-butyl-4-hydroxy-7-{[4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145270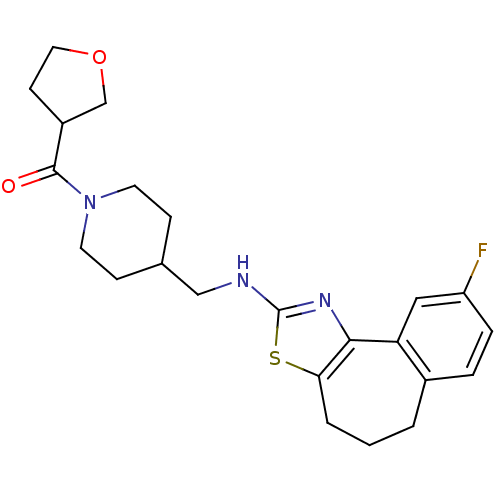 (CHEMBL81994 | {4-[(9-Fluoro-5,6-dihydro-4H-3-thia-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18350 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18339 ((2S,4S,5S,7S)-5-amino-N-[3-(dimethylcarbamoyl)prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110383 (US8613914, 157 | US9206232, 157) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18335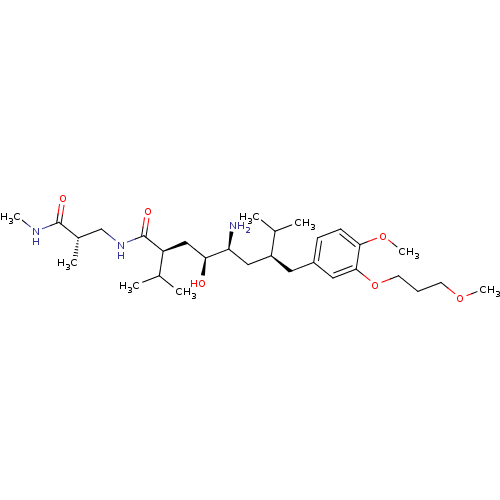 ((2S,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110397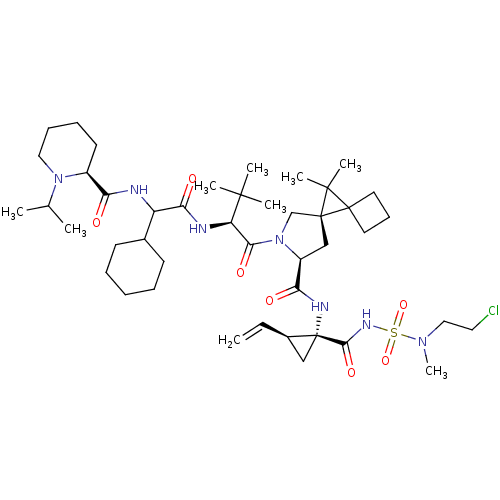 (US8613914, 171 | US9206232, 171) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18333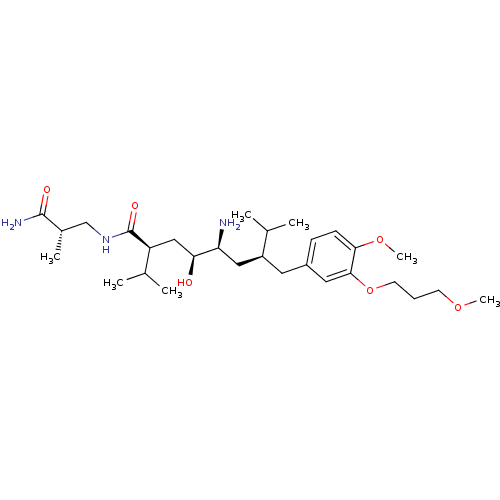 ((2S,4S,5S,7S)-5-amino-N-[(2S)-2-carbamoyl-2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110277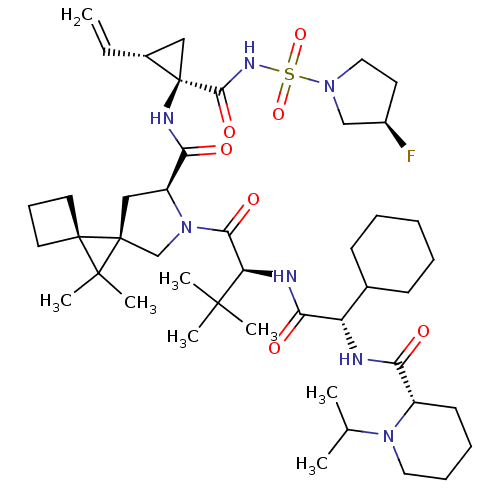 (US8613914, 45 | US9206232, 45) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18331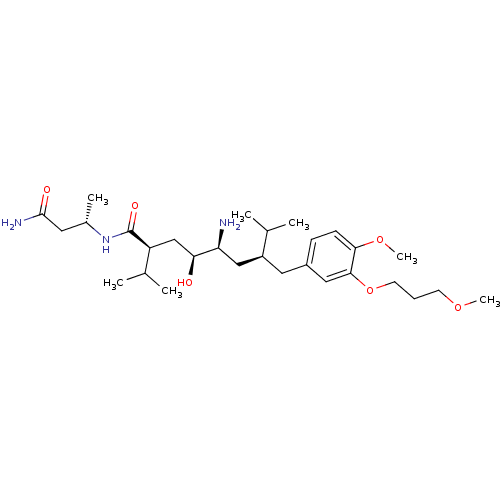 ((2S,4S,5S,7S)-5-amino-N-[(2S)-1-carbamoylpropan-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18322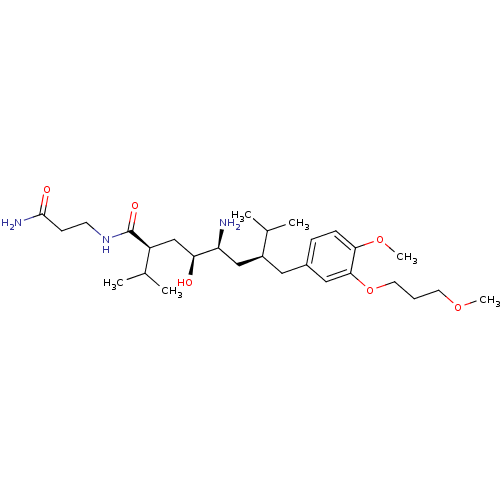 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoylethyl)-4-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110294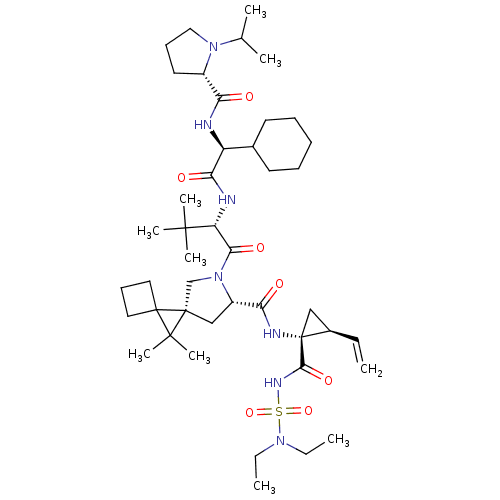 (US8613914, 62 | US9206232, 62) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18321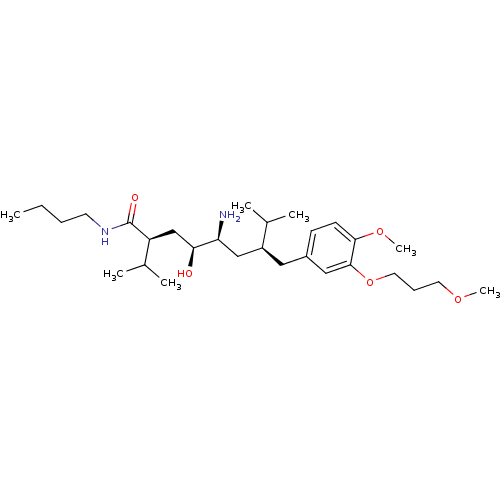 ((2S,4S,5S,7S)-5-amino-N-butyl-4-hydroxy-7-{[4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110294 (US8613914, 62 | US9206232, 62) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 897 total ) | Next | Last >> |