

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM162123 (US9051279, 106) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human MDM2 by TR-FRET assay | J Med Chem 58: 6348-58 (2015) Article DOI: 10.1021/acs.jmedchem.5b00810 BindingDB Entry DOI: 10.7270/Q2HQ41QV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50229787 ((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human MDM2 by TR-FRET assay | J Med Chem 58: 6348-58 (2015) Article DOI: 10.1021/acs.jmedchem.5b00810 BindingDB Entry DOI: 10.7270/Q2HQ41QV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Canis lupus familiaris) | BDBM50229787 ((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of dog MDM2 by TR-FRET assay | J Med Chem 58: 6348-58 (2015) Article DOI: 10.1021/acs.jmedchem.5b00810 BindingDB Entry DOI: 10.7270/Q2HQ41QV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Mus musculus) | BDBM50229787 ((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mouse MDM2 by TR-FRET assay | J Med Chem 58: 6348-58 (2015) Article DOI: 10.1021/acs.jmedchem.5b00810 BindingDB Entry DOI: 10.7270/Q2HQ41QV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Canis lupus familiaris) | BDBM162123 (US9051279, 106) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of dog MDM2 by TR-FRET assay | J Med Chem 58: 6348-58 (2015) Article DOI: 10.1021/acs.jmedchem.5b00810 BindingDB Entry DOI: 10.7270/Q2HQ41QV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Mus musculus) | BDBM162123 (US9051279, 106) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mouse MDM2 by TR-FRET assay | J Med Chem 58: 6348-58 (2015) Article DOI: 10.1021/acs.jmedchem.5b00810 BindingDB Entry DOI: 10.7270/Q2HQ41QV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50554937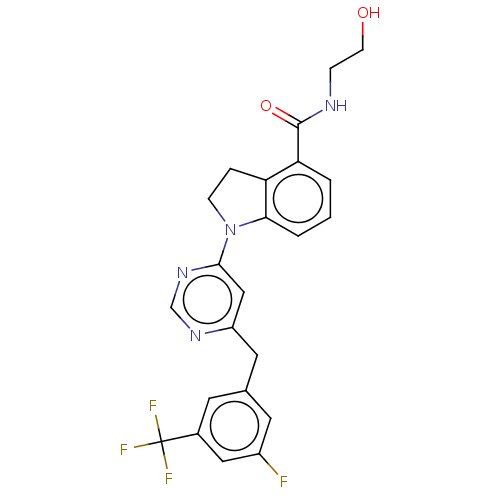 (CHEMBL4780654 | US20240059655, Compound PW0787) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to 5-HT2C (unknown origin) assessed as inhibition of ligand binding | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01498 BindingDB Entry DOI: 10.7270/Q22N55XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129810 (US8815926, 89) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129937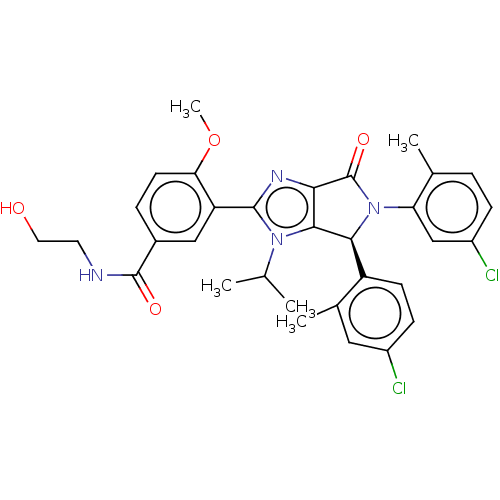 (US8815926, 218) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM130042 (US8815926, 325) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [18-26]/E3 ubiquitin-protein ligase Mdm2 [2-188] (Homo sapiens (Human)) | BDBM236338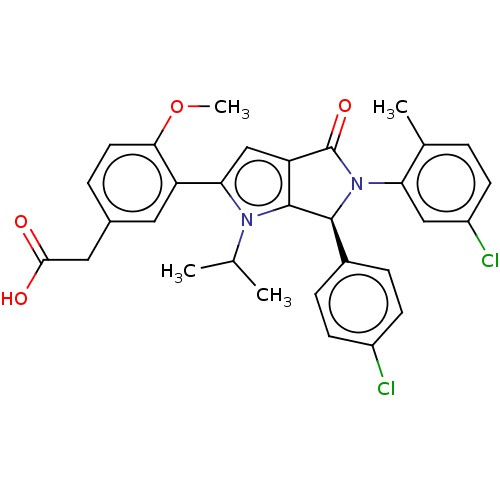 (US9365576, 91) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0610 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description Assay 2: The test is performed in white 384-well plates (Greiner Bio-One, reference 781207) in a total volume of 60 μL by adding 1 μL of compou... | US Patent US9365576 (2016) BindingDB Entry DOI: 10.7270/Q2DN43XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [18-26]/E3 ubiquitin-protein ligase Mdm2 [2-188] (Homo sapiens (Human)) | BDBM236270 (US9365576, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0650 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description Assay 2: The test is performed in white 384-well plates (Greiner Bio-One, reference 781207) in a total volume of 60 μL by adding 1 μL of compou... | US Patent US9365576 (2016) BindingDB Entry DOI: 10.7270/Q2DN43XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [18-26]/E3 ubiquitin-protein ligase Mdm2 [2-188] (Homo sapiens (Human)) | BDBM236261 (US9365576, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0680 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description Assay 2: The test is performed in white 384-well plates (Greiner Bio-One, reference 781207) in a total volume of 60 μL by adding 1 μL of compou... | US Patent US9365576 (2016) BindingDB Entry DOI: 10.7270/Q2DN43XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM143673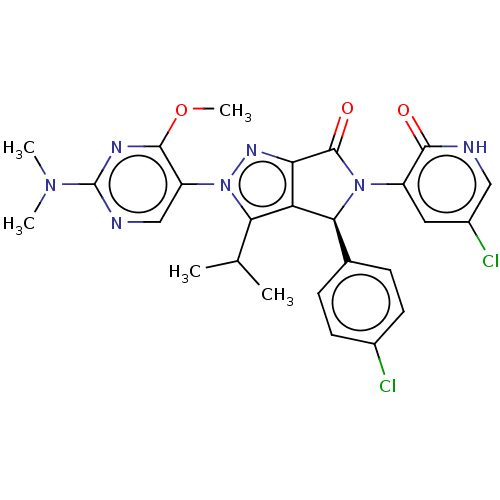 (US8969341, 180) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8969341 (2015) BindingDB Entry DOI: 10.7270/Q2K35SC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [18-26]/E3 ubiquitin-protein ligase Mdm2 [2-188] (Homo sapiens (Human)) | BDBM236476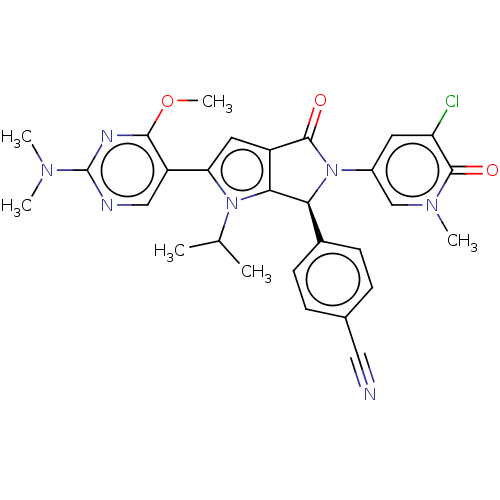 (US9365576, 232) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0690 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description Assay 2: The test is performed in white 384-well plates (Greiner Bio-One, reference 781207) in a total volume of 60 μL by adding 1 μL of compou... | US Patent US9365576 (2016) BindingDB Entry DOI: 10.7270/Q2DN43XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129796 (US8815926, 75) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129730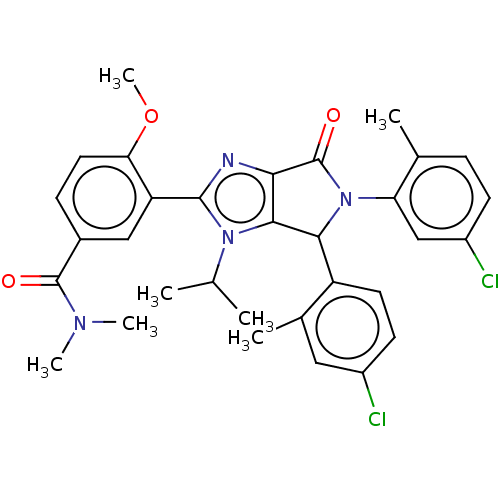 (US8815926, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129738 (US8815926, 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [18-26]/E3 ubiquitin-protein ligase Mdm2 [2-188] (Homo sapiens (Human)) | BDBM236471 (US9365576, 227) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0710 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description Assay 2: The test is performed in white 384-well plates (Greiner Bio-One, reference 781207) in a total volume of 60 μL by adding 1 μL of compou... | US Patent US9365576 (2016) BindingDB Entry DOI: 10.7270/Q2DN43XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50467282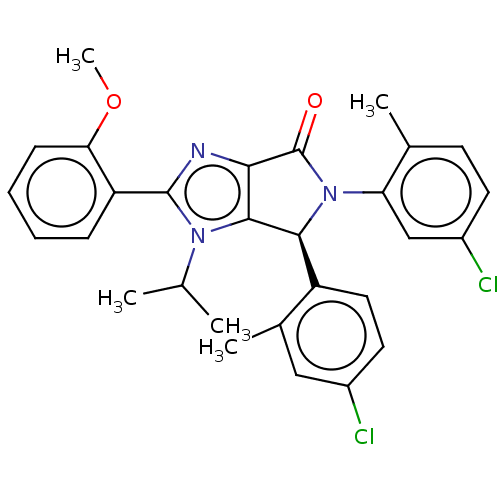 (CHEMBL4290086) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Cy5-labeled p53 derived TFSDLWKLL peptide binding to C-terminal biotin-labelled human MDM2 (2 to 188 residues) by TR-FRET assay | Bioorg Med Chem Lett 28: 3404-3408 (2018) Article DOI: 10.1016/j.bmcl.2018.08.027 BindingDB Entry DOI: 10.7270/Q2C82D0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [18-26]/E3 ubiquitin-protein ligase Mdm2 [2-188] (Homo sapiens (Human)) | BDBM236267 (US9365576, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description Assay 2: The test is performed in white 384-well plates (Greiner Bio-One, reference 781207) in a total volume of 60 μL by adding 1 μL of compou... | US Patent US9365576 (2016) BindingDB Entry DOI: 10.7270/Q2DN43XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [18-26]/E3 ubiquitin-protein ligase Mdm2 [2-188] (Homo sapiens (Human)) | BDBM236473 (US9365576, 229) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description Assay 2: The test is performed in white 384-well plates (Greiner Bio-One, reference 781207) in a total volume of 60 μL by adding 1 μL of compou... | US Patent US9365576 (2016) BindingDB Entry DOI: 10.7270/Q2DN43XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [18-26]/E3 ubiquitin-protein ligase Mdm2 [2-188] (Homo sapiens (Human)) | BDBM236435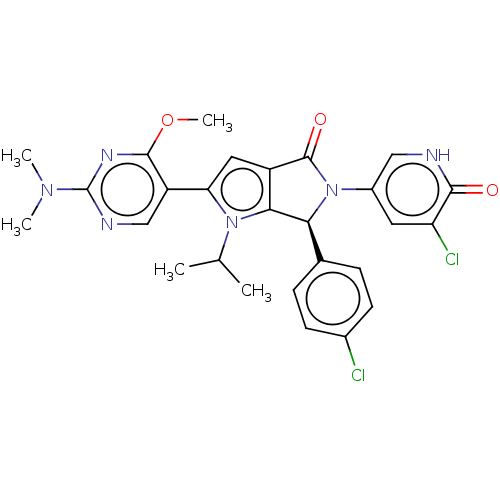 (US9365576, 188) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description Assay 2: The test is performed in white 384-well plates (Greiner Bio-One, reference 781207) in a total volume of 60 μL by adding 1 μL of compou... | US Patent US9365576 (2016) BindingDB Entry DOI: 10.7270/Q2DN43XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129966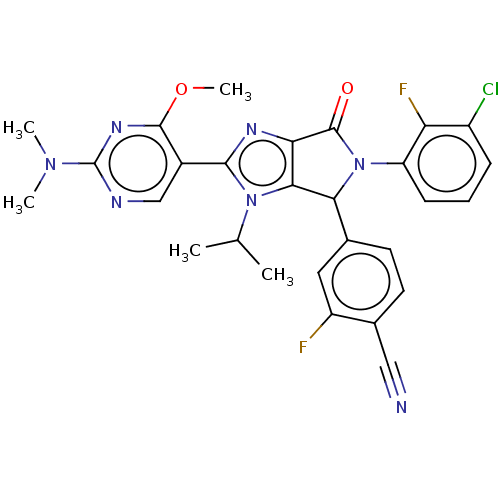 (US8815926, 247) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129945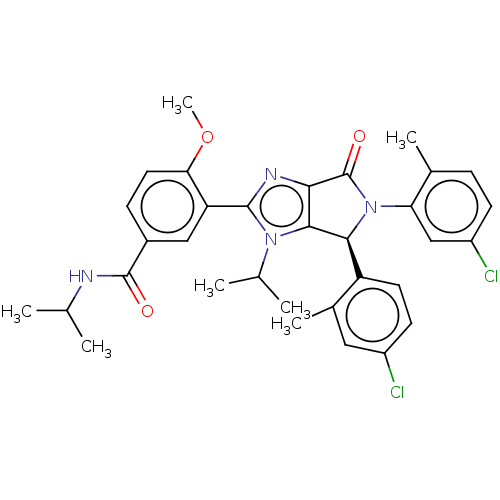 (US8815926, 226) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129796 (US8815926, 75) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Cy5-labeled p53 derived TFSDLWKLL peptide binding to C-terminal biotin-labelled human MDM2 (2 to 188 residues) by TR-FRET assay | Bioorg Med Chem Lett 28: 3404-3408 (2018) Article DOI: 10.1016/j.bmcl.2018.08.027 BindingDB Entry DOI: 10.7270/Q2C82D0H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129947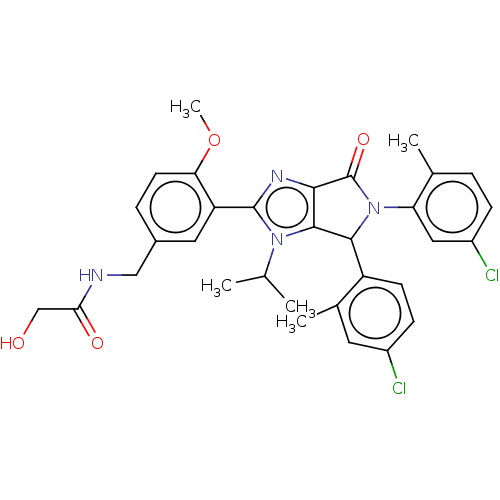 (US8815926, 228) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129892 (US8815926, 173) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [18-26]/E3 ubiquitin-protein ligase Mdm2 [2-188] (Homo sapiens (Human)) | BDBM236336 (US9365576, 89) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description Assay 2: The test is performed in white 384-well plates (Greiner Bio-One, reference 781207) in a total volume of 60 μL by adding 1 μL of compou... | US Patent US9365576 (2016) BindingDB Entry DOI: 10.7270/Q2DN43XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM130000 (US8815926, 281) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [18-26]/E3 ubiquitin-protein ligase Mdm2 [2-188] (Homo sapiens (Human)) | BDBM236368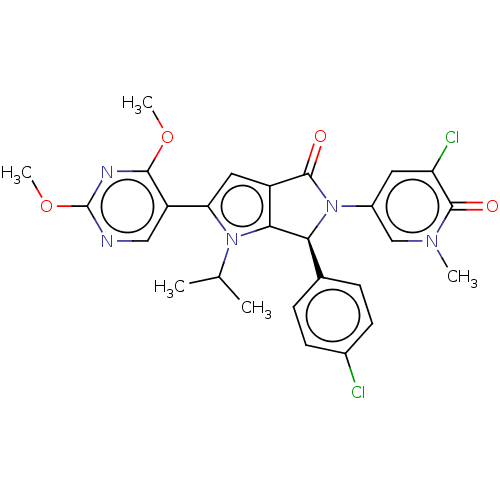 (US9365576, 121 | US9365576, 218) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0870 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description Assay 2: The test is performed in white 384-well plates (Greiner Bio-One, reference 781207) in a total volume of 60 μL by adding 1 μL of compou... | US Patent US9365576 (2016) BindingDB Entry DOI: 10.7270/Q2DN43XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM130035 (US8815926, 317) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129737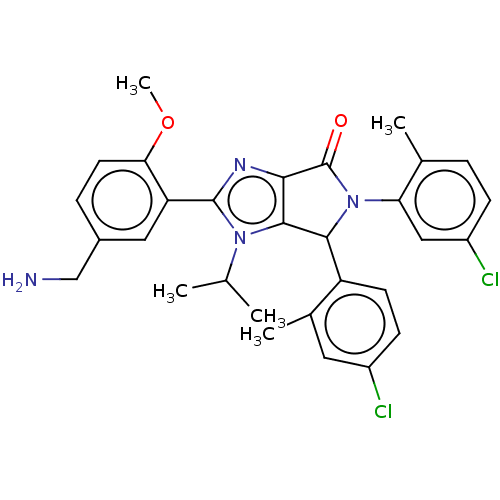 (US8815926, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129838 (US8815926, 117) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129928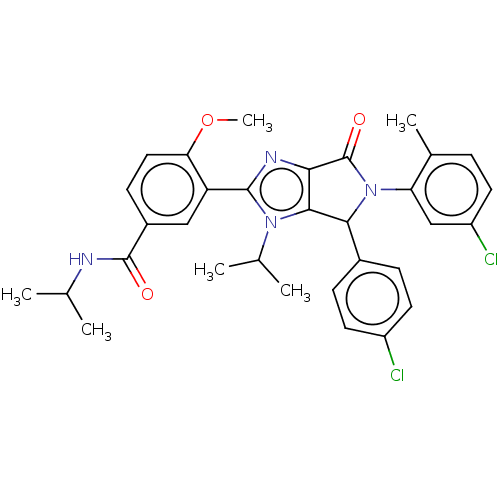 (US8815926, 209) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129942 (US8815926, 223) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [18-26]/E3 ubiquitin-protein ligase Mdm2 [2-188] (Homo sapiens (Human)) | BDBM236279 (US9365576, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0940 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description Assay 2: The test is performed in white 384-well plates (Greiner Bio-One, reference 781207) in a total volume of 60 μL by adding 1 μL of compou... | US Patent US9365576 (2016) BindingDB Entry DOI: 10.7270/Q2DN43XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [18-26]/E3 ubiquitin-protein ligase Mdm2 [2-188] (Homo sapiens (Human)) | BDBM236265 (US9365576, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0950 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description Assay 2: The test is performed in white 384-well plates (Greiner Bio-One, reference 781207) in a total volume of 60 μL by adding 1 μL of compou... | US Patent US9365576 (2016) BindingDB Entry DOI: 10.7270/Q2DN43XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM143676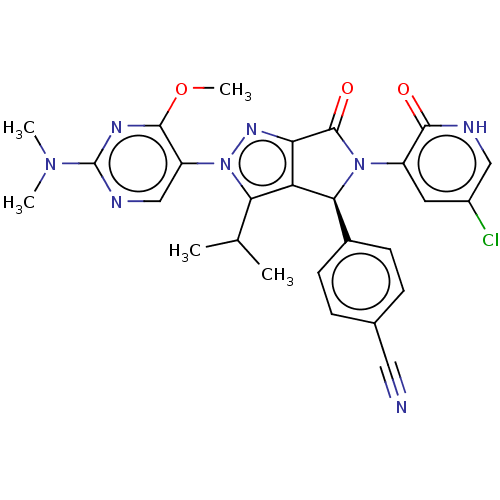 (US8969341, 183) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8969341 (2015) BindingDB Entry DOI: 10.7270/Q2K35SC8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17945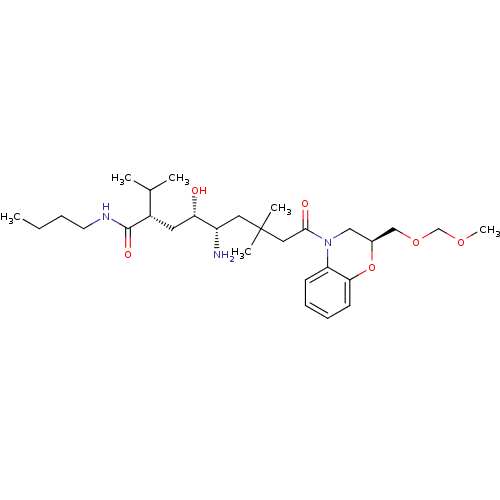 ((2S,4S,5S)-5-amino-N-butyl-4-hydroxy-9-[(2S)-2-[(m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description In vitro potencies of compounds against purified human recombinant renin were determined by its cleavage of substrate angiotensinogen. The angiotensi... | Chem Biol 7: 493-504 (2000) Article DOI: 10.1016/S1074-5521(00)00134-4 BindingDB Entry DOI: 10.7270/Q2V40SGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129884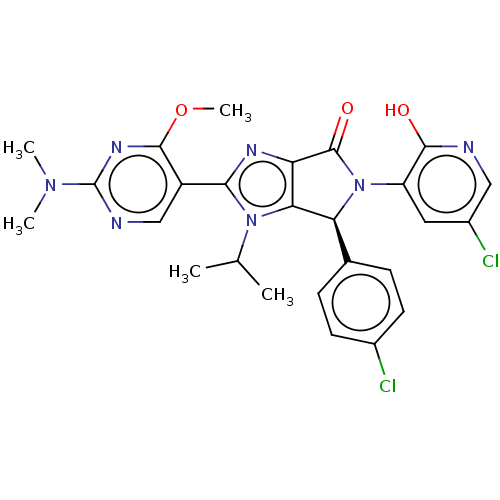 (US8815926, 164) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129894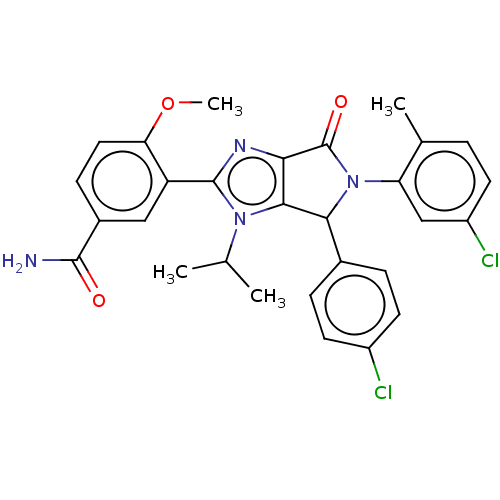 (US8815926, 175) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM143548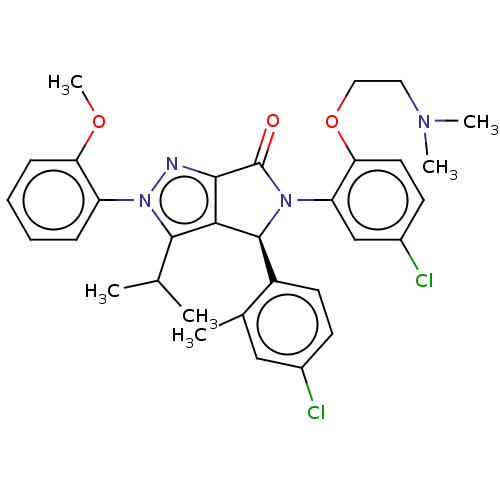 (US8969341, 143) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 0.101 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8969341 (2015) BindingDB Entry DOI: 10.7270/Q2K35SC8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM130001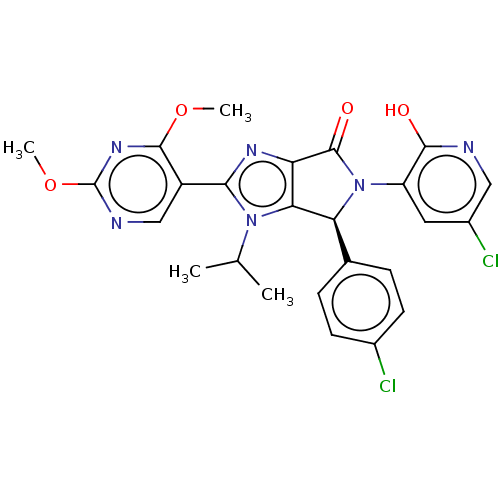 (US8815926, 282) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.102 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM143574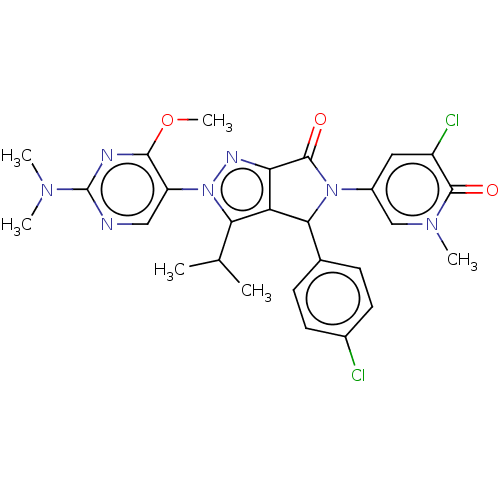 (US8969341, 149) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.103 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8969341 (2015) BindingDB Entry DOI: 10.7270/Q2K35SC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [18-26]/E3 ubiquitin-protein ligase Mdm2 [2-188] (Homo sapiens (Human)) | BDBM236308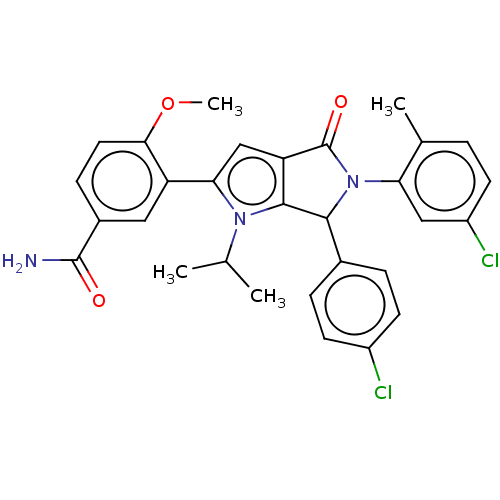 (US9365576, 61) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.104 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description Assay 2: The test is performed in white 384-well plates (Greiner Bio-One, reference 781207) in a total volume of 60 μL by adding 1 μL of compou... | US Patent US9365576 (2016) BindingDB Entry DOI: 10.7270/Q2DN43XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [18-26]/E3 ubiquitin-protein ligase Mdm2 [2-188] (Homo sapiens (Human)) | BDBM236275 (US9365576, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.106 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description Assay 2: The test is performed in white 384-well plates (Greiner Bio-One, reference 781207) in a total volume of 60 μL by adding 1 μL of compou... | US Patent US9365576 (2016) BindingDB Entry DOI: 10.7270/Q2DN43XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [18-26]/E3 ubiquitin-protein ligase Mdm2 [2-188] (Homo sapiens (Human)) | BDBM236370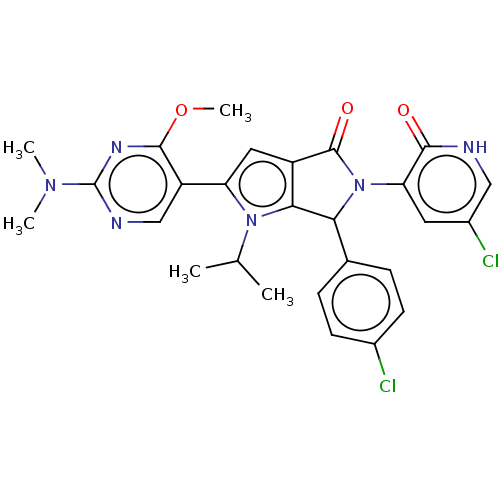 (US9365576, 123) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.106 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description Assay 2: The test is performed in white 384-well plates (Greiner Bio-One, reference 781207) in a total volume of 60 μL by adding 1 μL of compou... | US Patent US9365576 (2016) BindingDB Entry DOI: 10.7270/Q2DN43XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [18-26]/E3 ubiquitin-protein ligase Mdm2 [2-188] (Homo sapiens (Human)) | BDBM236368 (US9365576, 121 | US9365576, 218) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.106 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description Assay 2: The test is performed in white 384-well plates (Greiner Bio-One, reference 781207) in a total volume of 60 μL by adding 1 μL of compou... | US Patent US9365576 (2016) BindingDB Entry DOI: 10.7270/Q2DN43XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [18-26]/E3 ubiquitin-protein ligase Mdm2 [2-188] (Homo sapiens (Human)) | BDBM236481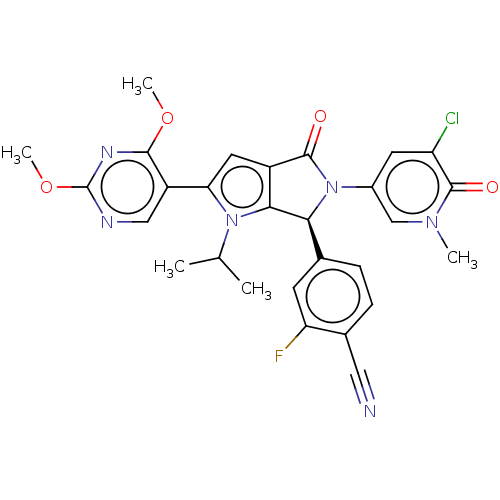 (US9365576, 237) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.107 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description Assay 2: The test is performed in white 384-well plates (Greiner Bio-One, reference 781207) in a total volume of 60 μL by adding 1 μL of compou... | US Patent US9365576 (2016) BindingDB Entry DOI: 10.7270/Q2DN43XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1933 total ) | Next | Last >> |