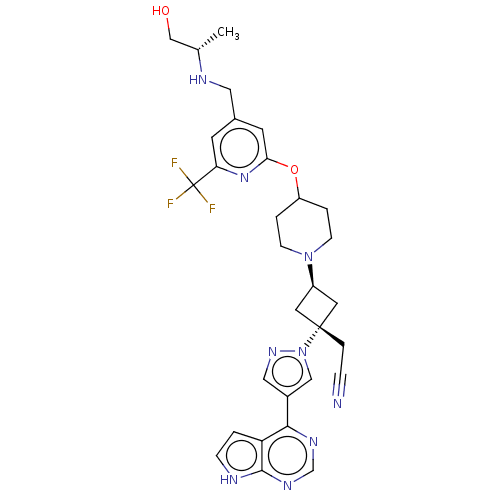
| Synonyms: | US10053465, 23 | US10065963, Compound 23 | US10125150, Example 23 | US10336759, # 23 | US10479803, Example 23 | US10519163, Example 23 | US10675284, Example 23 | US11084822, Example 23 | US11130767, # 23 | US11136326, Example 23 | US11304949, Compound 23 | US11324749, Comp. No. 23 | US11406640, Comp. No. 23 | US11596632, Comp. No. 23 | US11918581, Compound 23 | US20240058343, Compound 23 | US9732097, Example 23 | {trans-3-(4-{[4- ({[(1S)-2-hydroxy-1- methylethyl]amino} methyl)-6- (trifluoromethyl) pyridin-2- yl]oxy}piperidin-1- yl)-1-[4-(7H- pyrrolo[2,3- d]pyrimidin-4-yl)- 1H-pyrazol-1- yl]cyclobutyl} acetonitrile |
|---|
| SMILES | C[C@@H](CO)NCc1cc(OC2CCN(CC2)[C@H]2C[C@@](CC#N)(C2)n2cc(cn2)-c2ncnc3[nH]ccc23)nc(c1)C(F)(F)F |r,wU:18.24,1.0,wD:16.16,(-2.38,11.91,;-.89,11.51,;.2,12.6,;1.69,12.2,;-.49,10.02,;-1.58,8.93,;-1.18,7.44,;-2.27,6.36,;-1.87,4.87,;-2.96,3.78,;-2.56,2.29,;-3.65,1.2,;-3.25,-.28,;-1.77,-.68,;-.68,.41,;-1.08,1.89,;-1.37,-2.17,;-2.14,-3.5,;-.8,-4.27,;.68,-4.67,;1.77,-3.58,;2.86,-2.49,;-.03,-2.94,;-1.57,-5.61,;-1.1,-7.07,;-2.34,-7.98,;-3.59,-7.07,;-3.11,-5.61,;-2.34,-9.52,;-3.68,-10.29,;-3.68,-11.83,;-2.34,-12.6,;-1.01,-11.83,;.45,-12.3,;1.36,-11.06,;.45,-9.81,;-1.01,-10.29,;-.39,4.47,;.7,5.56,;.3,7.05,;2.19,5.16,;3.28,6.25,;2.59,3.67,;3.68,4.76,)| |
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Zhou, J; Qiao, L; Weng, L Process for the synthesis of a phosphoinositide 3-kinase inhibitor US Patent US9732097 Publication Date 8/15/2017
Zhou, J; Qiao, L; Weng, L Process for the synthesis of a phosphoinositide 3-kinase inhibitor US Patent US9732097 Publication Date 8/15/2017