

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Tyrosine-protein kinase Mer | ||
| Ligand | BDBM336466 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | Inhibition Assay | ||
| IC50 | <10±n/a nM | ||
| Citation |  Wang, X; Liu, J; Yang, C; Zhang, W; Frye, S; Kireev, D Pyrazolopyrimidine compounds for the treatment of cancer US Patent US9744172 Publication Date 8/29/2017 Wang, X; Liu, J; Yang, C; Zhang, W; Frye, S; Kireev, D Pyrazolopyrimidine compounds for the treatment of cancer US Patent US9744172 Publication Date 8/29/2017 | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Tyrosine-protein kinase Mer | |||
| Name: | Tyrosine-protein kinase Mer | ||
| Synonyms: | MER | MER intracellular domain/EGFR extracellular domain chimera | MERTK | MERTK_HUMAN | Proto-oncogene c-Mer | Proto-oncogene tyrosine-protein kinase MER | Receptor tyrosine kinase MerTK | Tyrosine-protein kinase Mer | ||
| Type: | PROTEIN | ||
| Mol. Mass.: | 110234.77 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | ChEMBL_1498723 | ||
| Residue: | 999 | ||
| Sequence: |
| ||
| BDBM336466 | |||
| n/a | |||
| Name | BDBM336466 | ||
| Synonyms: | US9744172, Compound UNC1265A | ||
| Type | Small organic molecule | ||
| Emp. Form. | C30H44N8O4S | ||
| Mol. Mass. | 612.787 | ||
| SMILES | CCCCNc1ncc2c(nn(C3CC[C@H](O)CC3)c2n1)-c1ccc(cc1)S(=O)(=O)NCCC(=O)NC1CCN(C)CC1 |r,wD:15.15,(-9.97,-6.6,;-8.64,-5.83,;-7.3,-6.6,;-5.97,-5.83,;-4.63,-6.6,;-3.3,-5.83,;-3.3,-4.29,;-1.97,-3.52,;-.63,-4.29,;.83,-3.81,;1.74,-5.06,;.83,-6.31,;1.23,-7.79,;2.72,-8.19,;3.12,-9.68,;2.03,-10.77,;2.43,-12.26,;.54,-10.37,;.14,-8.88,;-.63,-5.83,;-1.97,-6.6,;1.23,-2.33,;2.72,-1.93,;3.12,-.44,;2.03,.65,;.54,.25,;.14,-1.24,;2.43,2.14,;.94,2.53,;2.03,3.62,;3.91,2.53,;4.31,4.02,;5.8,4.42,;6.2,5.91,;5.11,7,;7.68,6.31,;8.08,7.79,;9.57,8.19,;9.97,9.68,;8.88,10.77,;9.28,12.26,;7.39,10.37,;6.99,8.88,)| | ||
| Structure | 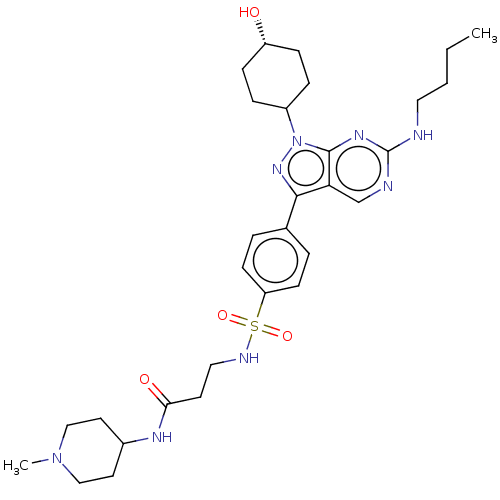
| ||