

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Cyclin-dependent kinase 12 | ||
| Ligand | BDBM50367707 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_1735959 (CHEMBL4151495) | ||
| IC50 | 63±n/a nM | ||
| Citation |  Ito, M; Tanaka, T; Toita, A; Uchiyama, N; Kokubo, H; Morishita, N; Klein, MG; Zou, H; Murakami, M; Kondo, M; Sameshima, T; Araki, S; Endo, S; Kawamoto, T; Morin, GB; Aparicio, SA; Nakanishi, A; Maezaki, H; Imaeda, Y Discovery of 3-Benzyl-1-( trans-4-((5-cyanopyridin-2-yl)amino)cyclohexyl)-1-arylurea Derivatives as Novel and Selective Cyclin-Dependent Kinase 12 (CDK12) Inhibitors. J Med Chem61:7710-7728 (2018) [PubMed] Article Ito, M; Tanaka, T; Toita, A; Uchiyama, N; Kokubo, H; Morishita, N; Klein, MG; Zou, H; Murakami, M; Kondo, M; Sameshima, T; Araki, S; Endo, S; Kawamoto, T; Morin, GB; Aparicio, SA; Nakanishi, A; Maezaki, H; Imaeda, Y Discovery of 3-Benzyl-1-( trans-4-((5-cyanopyridin-2-yl)amino)cyclohexyl)-1-arylurea Derivatives as Novel and Selective Cyclin-Dependent Kinase 12 (CDK12) Inhibitors. J Med Chem61:7710-7728 (2018) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Cyclin-dependent kinase 12 | |||
| Name: | Cyclin-dependent kinase 12 | ||
| Synonyms: | 2.7.11.22 | 2.7.11.23 | CDC2-related protein kinase 7 | CDK12 | CDK12_HUMAN | CRK7 | CRKRS | Cdc2-related kinase, arginine/serine-rich | Cell division cycle 2-related protein kinase 7 | Cell division protein kinase 12 | Cyclin-dependent kinase 12 | KIAA0904 | hCDK12 | ||
| Type: | PROTEIN | ||
| Mol. Mass.: | 164218.64 | ||
| Organism: | Homo sapiens | ||
| Description: | ChEMBL_117739 | ||
| Residue: | 1490 | ||
| Sequence: |
| ||
| BDBM50367707 | |||
| n/a | |||
| Name | BDBM50367707 | ||
| Synonyms: | CHEMBL4167916 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C26H27N5O | ||
| Mol. Mass. | 425.5255 | ||
| SMILES | O=C(NCc1ccccc1)N([C@H]1CC[C@@H](CC1)Nc1ccc(cn1)C#N)c1ccccc1 |r,wU:11.11,wD:14.18,(25.33,-38.6,;25.33,-37.05,;26.66,-36.28,;27.99,-37.05,;29.32,-36.28,;29.32,-34.75,;30.66,-33.97,;31.99,-34.75,;31.99,-36.28,;30.66,-37.05,;23.99,-36.28,;22.66,-37.05,;22.66,-38.6,;21.33,-39.36,;20,-38.6,;20,-37.05,;21.33,-36.28,;18.67,-39.36,;17.34,-38.6,;16,-39.36,;14.67,-38.6,;14.67,-37.05,;16,-36.28,;17.34,-37.05,;13.34,-36.28,;12,-35.51,;23.99,-34.75,;22.66,-33.97,;22.66,-32.44,;23.99,-31.67,;25.33,-32.44,;25.33,-33.97,)| | ||
| Structure | 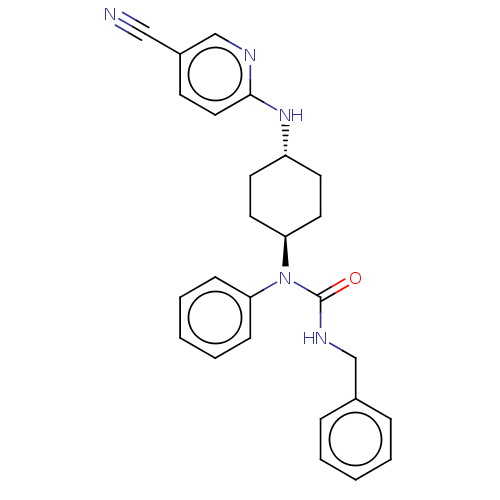
| ||