 Found 2543 hits with Last Name = 'endo' and Initial = 's'
Found 2543 hits with Last Name = 'endo' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM22416

((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...)Show SMILES Fc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 Show InChI InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]RTI55 binding from human wild type SERT |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113533
BindingDB Entry DOI: 10.7270/Q24T6P6C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XII
(Homo sapiens (Human)) | BDBM50229873
(CHEMBL4088217)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CSCCC(=O)N3CN(CN(C3)C(=O)CCSC[C@H](NC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](Cc3ccc(F)cc3)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N3CCC[C@H]3C(=O)N2)C(=O)CCSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C82H137FN32O18S3/c1-44(2)35-54-70(125)101-51(14-8-29-99-81(92)93)68(123)110-58(73(128)105-53(77(132)133)15-9-30-100-82(94)95)39-135-33-24-63(118)113-41-112-42-114(43-113)64(119)25-34-136-40-59(72(127)103-49(12-6-27-97-79(88)89)66(121)104-52(69(124)106-54)21-22-61(85)116)111-75(130)60-16-10-31-115(60)76(131)56(36-45(3)4)108-67(122)50(13-7-28-98-80(90)91)102-71(126)55(37-46-17-19-47(83)20-18-46)107-74(129)57(38-134-32-23-62(112)117)109-65(120)48(84)11-5-26-96-78(86)87/h17-20,44-45,48-60H,5-16,21-43,84H2,1-4H3,(H2,85,116)(H,101,125)(H,102,126)(H,103,127)(H,104,121)(H,105,128)(H,106,124)(H,107,129)(H,108,122)(H,109,120)(H,110,123)(H,111,130)(H,132,133)(H4,86,87,96)(H4,88,89,97)(H4,90,91,98)(H4,92,93,99)(H4,94,95,100)/t48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Polytechnique F�d�rale de Lausanne (EPFL)
Curated by ChEMBL
| Assay Description
Inhibition of human beta factor 12a using fluorogenic substrate Boc-Gln-Gly-Arg-AMC preincubated for 10 mins followed by addition of substrate measur... |
J Med Chem 60: 1151-1158 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01548
BindingDB Entry DOI: 10.7270/Q26D5W8V |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330427
(5-Chloro-4-hydroxybiphenyl-3-carboxylic acid | CHE...)Show InChI InChI=1S/C13H9ClO3/c14-11-7-9(8-4-2-1-3-5-8)6-10(12(11)15)13(16)17/h1-7,15H,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant AKR1C1 Phe311Leu mutant by fluorescence assay |
Bioorg Med Chem Lett 21: 2564-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.076
BindingDB Entry DOI: 10.7270/Q20G3NZ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50048803

(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methylspiperone from human D2 long receptor expressed in HEK293 cells measured after 1 hr by liquid scintillation counter method |
Eur J Med Chem 179: 1-15 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.001
BindingDB Entry DOI: 10.7270/Q2S185TZ |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330427

(5-Chloro-4-hydroxybiphenyl-3-carboxylic acid | CHE...)Show InChI InChI=1S/C13H9ClO3/c14-11-7-9(8-4-2-1-3-5-8)6-10(12(11)15)13(16)17/h1-7,15H,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XII
(Homo sapiens (Human)) | BDBM50229867

(CHEMBL4098389)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CSCCC(=O)N3CN(CN(C3)C(=O)CCSC[C@H](NC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](Cc3ccc(F)cc3)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N3CCC[C@H]3C(=O)N2)C(=O)CCSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O |r| Show InChI InChI=1S/C93H147FN34O19S3/c1-50(2)39-63-81(139)113-61(19-11-34-111-93(105)106)79(137)123-68(83(141)116-60(18-10-33-110-92(103)104)78(136)121-66(88(146)147)42-53-43-112-57-15-6-5-13-55(53)57)45-149-37-28-73(131)126-47-125-48-127(49-126)74(132)29-38-150-46-69(84(142)115-58(16-8-31-108-90(99)100)76(134)117-62(80(138)118-63)25-26-71(96)129)124-86(144)70-20-12-35-128(70)87(145)65(40-51(3)4)120-77(135)59(17-9-32-109-91(101)102)114-82(140)64(41-52-21-23-54(94)24-22-52)119-85(143)67(44-148-36-27-72(125)130)122-75(133)56(95)14-7-30-107-89(97)98/h5-6,13,15,21-24,43,50-51,56,58-70,112H,7-12,14,16-20,25-42,44-49,95H2,1-4H3,(H2,96,129)(H,113,139)(H,114,140)(H,115,142)(H,116,141)(H,117,134)(H,118,138)(H,119,143)(H,120,135)(H,121,136)(H,122,133)(H,123,137)(H,124,144)(H,146,147)(H4,97,98,107)(H4,99,100,108)(H4,101,102,109)(H4,103,104,110)(H4,105,106,111)/t56-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Polytechnique F�d�rale de Lausanne (EPFL)
Curated by ChEMBL
| Assay Description
Inhibition of human beta factor 12a using fluorogenic substrate Boc-Gln-Gly-Arg-AMC preincubated for 10 mins followed by addition of substrate measur... |
J Med Chem 60: 1151-1158 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01548
BindingDB Entry DOI: 10.7270/Q26D5W8V |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50048803

(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H] raclopride from human recombinant D2L receptor expressed in HEK293 cells measured after 1 hr by microbeta scintillation counting... |
Eur J Med Chem 170: 261-275 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.017
BindingDB Entry DOI: 10.7270/Q2222Z6Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50048803

(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methylspiperone from human D2 long receptor expressed in HEK293 cells measured after 1 hr by liquid scintillation counter method |
Eur J Med Chem 179: 1-15 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.001
BindingDB Entry DOI: 10.7270/Q2S185TZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50562710

(CHEMBL4759730)Show SMILES CN1CCN(CC1)c1cccc2n(ccc12)S(=O)(=O)c1ccccc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5-HT6 receptor incubated for 1 hr by microbeta counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112916
BindingDB Entry DOI: 10.7270/Q22R3WD3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50578580
(CHEMBL4848081)Show SMILES Brc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 |r| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]RTI55 binding from human wild type SERT |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113533
BindingDB Entry DOI: 10.7270/Q24T6P6C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330427

(5-Chloro-4-hydroxybiphenyl-3-carboxylic acid | CHE...)Show InChI InChI=1S/C13H9ClO3/c14-11-7-9(8-4-2-1-3-5-8)6-10(12(11)15)13(16)17/h1-7,15H,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant AKR1C1 Leu308Ala mutant by fluorescence assay |
Bioorg Med Chem Lett 21: 2564-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.076
BindingDB Entry DOI: 10.7270/Q20G3NZ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50442489

(CHEMBL2440417)Show SMILES COc1ccc(cc1)\N=c1/oc2cc(O)ccc2cc1C(=O)NCc1ccccc1 Show InChI InChI=1S/C24H20N2O4/c1-29-20-11-8-18(9-12-20)26-24-21(13-17-7-10-19(27)14-22(17)30-24)23(28)25-15-16-5-3-2-4-6-16/h2-14,27H,15H2,1H3,(H,25,28)/b26-24- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of wild-type human recombinant N-terminal His6-tagged AKR1B10 expressed in Escherichia coli using geraniol as substrate by dou... |
Bioorg Med Chem 21: 6378-84 (2013)
Article DOI: 10.1016/j.bmc.2013.08.059
BindingDB Entry DOI: 10.7270/Q25T3MX4 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330428
(5-Chloro-4-hydroxy-3'-methylbiphenyl-3-carboxylic ...)Show InChI InChI=1S/C14H11ClO3/c1-8-3-2-4-9(5-8)10-6-11(14(17)18)13(16)12(15)7-10/h2-7,16H,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330426
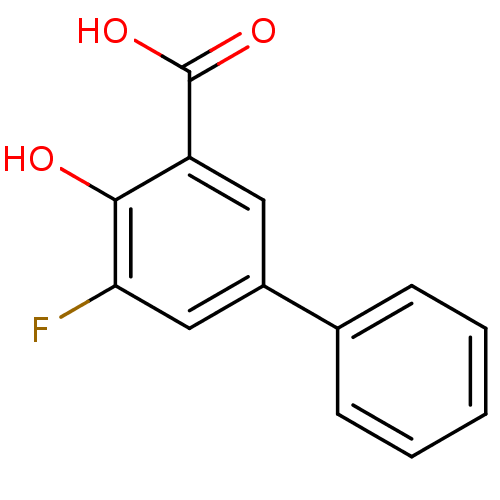
(5-Fluoro-4-hydroxybiphenyl-3-carboxylic acid | CHE...)Show InChI InChI=1S/C13H9FO3/c14-11-7-9(8-4-2-1-3-5-8)6-10(12(11)15)13(16)17/h1-7,15H,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330427

(5-Chloro-4-hydroxybiphenyl-3-carboxylic acid | CHE...)Show InChI InChI=1S/C13H9ClO3/c14-11-7-9(8-4-2-1-3-5-8)6-10(12(11)15)13(16)17/h1-7,15H,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant AKR1C1 Phe311Ala mutant by fluorescence assay |
Bioorg Med Chem Lett 21: 2564-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.076
BindingDB Entry DOI: 10.7270/Q20G3NZ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50330426

(5-Fluoro-4-hydroxybiphenyl-3-carboxylic acid | CHE...)Show InChI InChI=1S/C13H9FO3/c14-11-7-9(8-4-2-1-3-5-8)6-10(12(11)15)13(16)17/h1-7,15H,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | |
Coagulation factor XII
(Homo sapiens (Human)) | BDBM50229738
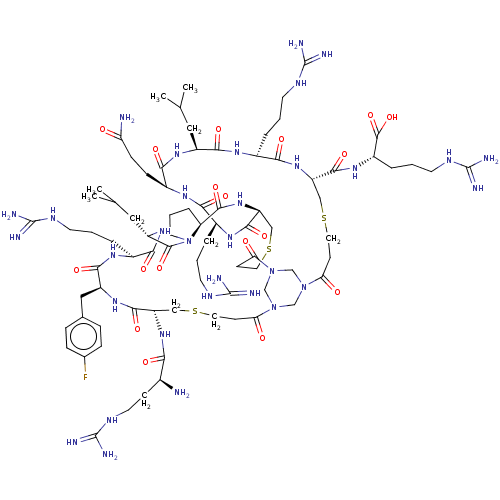
(CHEMBL4087027)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CSCCC(=O)N3CN(CN(C3)C(=O)CCSC[C@H](NC(=O)[C@@H](N)CCNC(N)=N)C(=O)N[C@@H](Cc3ccc(F)cc3)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N3CCC[C@H]3C(=O)N2)C(=O)CCSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C81H135FN32O18S3/c1-43(2)34-53-69(124)100-50(12-7-27-97-79(89)90)67(122)109-57(72(127)104-52(76(131)132)13-8-28-98-80(91)92)38-134-32-23-62(117)112-40-111-41-113(42-112)63(118)24-33-135-39-58(71(126)102-48(10-5-25-95-77(85)86)65(120)103-51(68(123)105-53)19-20-60(84)115)110-74(129)59-14-9-30-114(59)75(130)55(35-44(3)4)107-66(121)49(11-6-26-96-78(87)88)101-70(125)54(36-45-15-17-46(82)18-16-45)106-73(128)56(37-133-31-22-61(111)116)108-64(119)47(83)21-29-99-81(93)94/h15-18,43-44,47-59H,5-14,19-42,83H2,1-4H3,(H2,84,115)(H,100,124)(H,101,125)(H,102,126)(H,103,120)(H,104,127)(H,105,123)(H,106,128)(H,107,121)(H,108,119)(H,109,122)(H,110,129)(H,131,132)(H4,85,86,95)(H4,87,88,96)(H4,89,90,97)(H4,91,92,98)(H4,93,94,99)/t47-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Polytechnique F�d�rale de Lausanne (EPFL)
Curated by ChEMBL
| Assay Description
Inhibition of human beta factor 12a using fluorogenic substrate Boc-Gln-Gly-Arg-AMC preincubated for 10 mins followed by addition of substrate measur... |
J Med Chem 60: 1151-1158 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01548
BindingDB Entry DOI: 10.7270/Q26D5W8V |
More data for this
Ligand-Target Pair | |
Coagulation factor XII
(Homo sapiens (Human)) | BDBM50229872
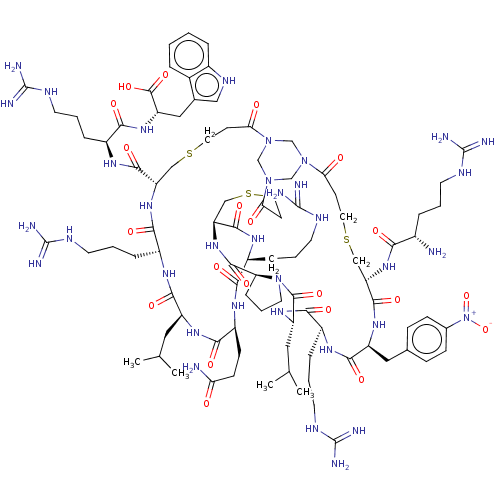
(CHEMBL4059973)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CSCCC(=O)N3CN(CN(C3)C(=O)CCSC[C@H](NC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](Cc3ccc(cc3)[N+]([O-])=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N3CCC[C@H]3C(=O)N2)C(=O)CCSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O |r| Show InChI InChI=1S/C93H147N35O21S3/c1-50(2)39-63-81(139)112-61(19-11-34-110-93(104)105)79(137)122-68(83(141)115-60(18-10-33-109-92(102)103)78(136)120-66(88(146)147)42-53-43-111-57-15-6-5-13-55(53)57)45-151-37-28-73(131)125-47-124-48-126(49-125)74(132)29-38-152-46-69(84(142)114-58(16-8-31-107-90(98)99)76(134)116-62(80(138)117-63)25-26-71(95)129)123-86(144)70-20-12-35-127(70)87(145)65(40-51(3)4)119-77(135)59(17-9-32-108-91(100)101)113-82(140)64(41-52-21-23-54(24-22-52)128(148)149)118-85(143)67(44-150-36-27-72(124)130)121-75(133)56(94)14-7-30-106-89(96)97/h5-6,13,15,21-24,43,50-51,56,58-70,111H,7-12,14,16-20,25-42,44-49,94H2,1-4H3,(H2,95,129)(H,112,139)(H,113,140)(H,114,142)(H,115,141)(H,116,134)(H,117,138)(H,118,143)(H,119,135)(H,120,136)(H,121,133)(H,122,137)(H,123,144)(H,146,147)(H4,96,97,106)(H4,98,99,107)(H4,100,101,108)(H4,102,103,109)(H4,104,105,110)/t56-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Polytechnique F�d�rale de Lausanne (EPFL)
Curated by ChEMBL
| Assay Description
Inhibition of human beta factor 12a using fluorogenic substrate Boc-Gln-Gly-Arg-AMC preincubated for 10 mins followed by addition of substrate measur... |
J Med Chem 60: 1151-1158 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01548
BindingDB Entry DOI: 10.7270/Q26D5W8V |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330427

(5-Chloro-4-hydroxybiphenyl-3-carboxylic acid | CHE...)Show InChI InChI=1S/C13H9ClO3/c14-11-7-9(8-4-2-1-3-5-8)6-10(12(11)15)13(16)17/h1-7,15H,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant AKR1C1 Leu308Val mutant by fluorescence assay |
Bioorg Med Chem Lett 21: 2564-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.076
BindingDB Entry DOI: 10.7270/Q20G3NZ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330432
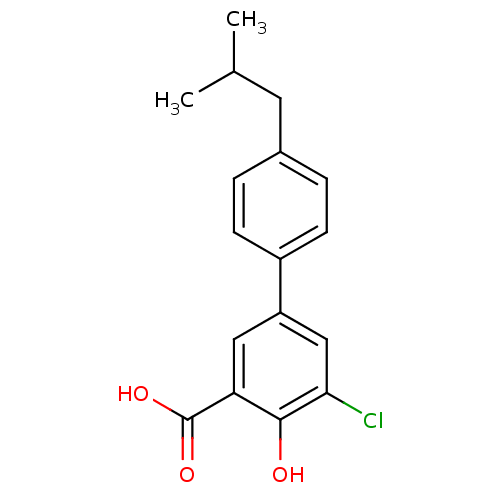
(5-Chloro-4-hydroxy-4'-isobutylbiphenyl-3-carboxyli...)Show InChI InChI=1S/C17H17ClO3/c1-10(2)7-11-3-5-12(6-4-11)13-8-14(17(20)21)16(19)15(18)9-13/h3-6,8-10,19H,7H2,1-2H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50504839

(CHEMBL4584504)Show SMILES Oc1ccc(cc1)-c1ccc(CNCCc2c[nH]c3cc(F)ccc23)o1 Show InChI InChI=1S/C21H19FN2O2/c22-16-3-7-19-15(12-24-20(19)11-16)9-10-23-13-18-6-8-21(26-18)14-1-4-17(25)5-2-14/h1-8,11-12,23-25H,9-10,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6R expressed in HEK293 cells after 1 hr by microbeta plate reader analysis |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50504838

(CHEMBL4437523)Show SMILES Oc1ccc(cc1)-c1ccc(CNCCc2c[nH]c3ccc(F)cc23)o1 Show InChI InChI=1S/C21H19FN2O2/c22-16-3-7-20-19(11-16)15(12-24-20)9-10-23-13-18-6-8-21(26-18)14-1-4-17(25)5-2-14/h1-8,11-12,23-25H,9-10,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6R expressed in HEK293 cells after 1 hr by microbeta plate reader analysis |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50513415

(CHEMBL4435010)Show SMILES Cl.COc1ccc2n(cc(-c3cnc(N)[nH]3)c2c1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C18H16N4O3S/c1-25-12-7-8-17-14(9-12)15(16-10-20-18(19)21-16)11-22(17)26(23,24)13-5-3-2-4-6-13/h2-11H,1H3,(H3,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells measured after 1 hr by liquid scintillation counter method |
Eur J Med Chem 179: 1-15 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.001
BindingDB Entry DOI: 10.7270/Q2S185TZ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50048803

(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D2R expressed in HEK293 cells after 1 hr by microbeta plate reader analysis |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330431

(4'-Butyl-5-chloro-4-hydroxybiphenyl-3-carboxylic a...)Show InChI InChI=1S/C17H17ClO3/c1-2-3-4-11-5-7-12(8-6-11)13-9-14(17(20)21)16(19)15(18)10-13/h5-10,19H,2-4H2,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241817
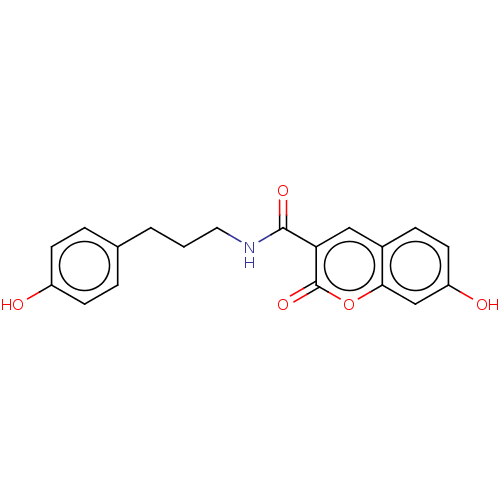
(CHEMBL4081954)Show InChI InChI=1S/C19H17NO5/c21-14-6-3-12(4-7-14)2-1-9-20-18(23)16-10-13-5-8-15(22)11-17(13)25-19(16)24/h3-8,10-11,21-22H,1-2,9H2,(H,20,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human AKR1B10 in presence of geraniol as substrate by Lineweaver-Burk plot method |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330429

(5-Chloro-4-hydroxy-4'-methylbiphenyl-3-carboxylic ...)Show InChI InChI=1S/C14H11ClO3/c1-8-2-4-9(5-3-8)10-6-11(14(17)18)13(16)12(15)7-10/h2-7,16H,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50362835
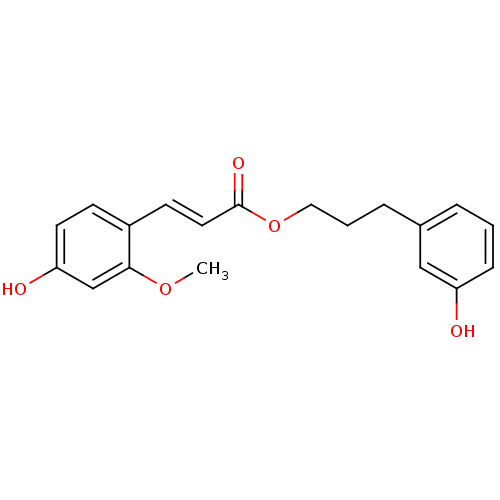
(CHEMBL1940400)Show InChI InChI=1S/C19H20O5/c1-23-18-13-17(21)9-7-15(18)8-10-19(22)24-11-3-5-14-4-2-6-16(20)12-14/h2,4,6-10,12-13,20-21H,3,5,11H2,1H3/b10-8+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition at human recombinant N-terminus His6-tagged AKR1B10 expressed in Escherichia coli BL21 DE3 assessed as inhibition of NADP+ lin... |
Eur J Med Chem 48: 321-9 (2012)
Article DOI: 10.1016/j.ejmech.2011.12.034
BindingDB Entry DOI: 10.7270/Q2TT4RDB |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50321717

(7-hydroxy-2-(4-methoxyphenylimino)-N-(pyridin-2-yl...)Show SMILES COc1ccc(cc1)\N=c1/oc2cc(O)ccc2cc1C(=O)Nc1ccccn1 Show InChI InChI=1S/C22H17N3O4/c1-28-17-9-6-15(7-10-17)24-22-18(21(27)25-20-4-2-3-11-23-20)12-14-5-8-16(26)13-19(14)29-22/h2-13,26H,1H3,(H,23,25,27)/b24-22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of dehydrogenase activity of N-terminal 6His-tagged AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as inhibition of geraniol deh... |
Bioorg Med Chem 18: 2485-90 (2010)
Article DOI: 10.1016/j.bmc.2010.02.050
BindingDB Entry DOI: 10.7270/Q2K35TTH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50513438
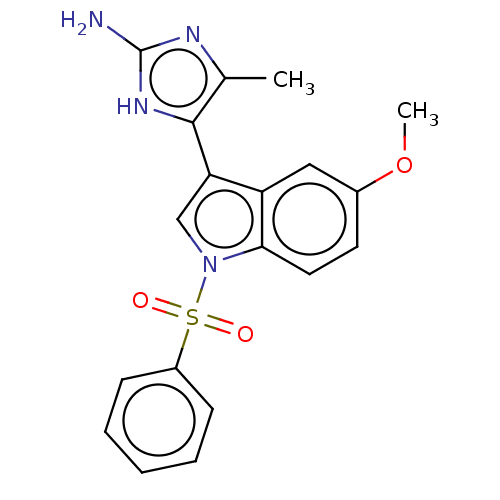
(CHEMBL4574931)Show SMILES Cl.COc1ccc2n(cc(-c3[nH]c(N)nc3C)c2c1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C19H18N4O3S/c1-12-18(22-19(20)21-12)16-11-23(17-9-8-13(26-2)10-15(16)17)27(24,25)14-6-4-3-5-7-14/h3-11H,1-2H3,(H3,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells measured after 1 hr by liquid scintillation counter method |
Eur J Med Chem 179: 1-15 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.001
BindingDB Entry DOI: 10.7270/Q2S185TZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50513432
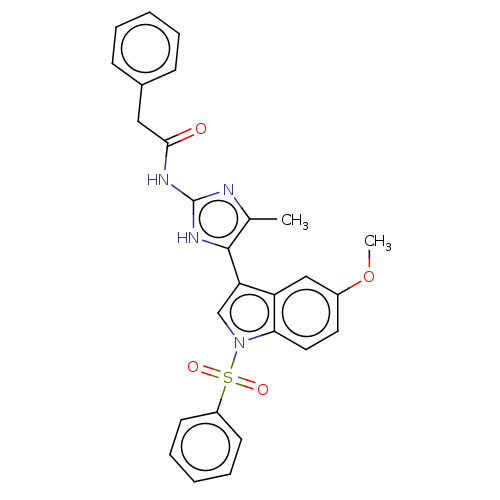
(CHEMBL4586990)Show SMILES COc1ccc2n(cc(-c3[nH]c(NC(=O)Cc4ccccc4)nc3C)c2c1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C27H24N4O4S/c1-18-26(30-27(28-18)29-25(32)15-19-9-5-3-6-10-19)23-17-31(24-14-13-20(35-2)16-22(23)24)36(33,34)21-11-7-4-8-12-21/h3-14,16-17H,15H2,1-2H3,(H2,28,29,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells measured after 1 hr by liquid scintillation counter method |
Eur J Med Chem 179: 1-15 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.001
BindingDB Entry DOI: 10.7270/Q2S185TZ |
More data for this
Ligand-Target Pair | |
Coagulation factor XII
(Homo sapiens (Human)) | BDBM50229758

(CHEMBL4102962)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CSCCC(=O)N3CN(CN(C3)C(=O)CCSC[C@H](NC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](Cc3cccnc3)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N3CCC[C@H]3C(=O)N2)C(=O)CCSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O |r| Show InChI InChI=1S/C92H147N35O19S3/c1-50(2)38-62-80(138)112-60(21-12-33-110-92(103)104)78(136)122-67(82(140)115-59(20-11-32-109-91(101)102)77(135)120-65(87(145)146)41-53-43-111-56-17-6-5-15-54(53)56)45-148-36-26-72(130)125-47-124-48-126(49-125)73(131)27-37-149-46-68(83(141)114-57(18-9-30-107-89(97)98)75(133)116-61(79(137)117-62)23-24-70(94)128)123-85(143)69-22-13-34-127(69)86(144)64(39-51(3)4)119-76(134)58(19-10-31-108-90(99)100)113-81(139)63(40-52-14-7-28-105-42-52)118-84(142)66(44-147-35-25-71(124)129)121-74(132)55(93)16-8-29-106-88(95)96/h5-7,14-15,17,28,42-43,50-51,55,57-69,111H,8-13,16,18-27,29-41,44-49,93H2,1-4H3,(H2,94,128)(H,112,138)(H,113,139)(H,114,141)(H,115,140)(H,116,133)(H,117,137)(H,118,142)(H,119,134)(H,120,135)(H,121,132)(H,122,136)(H,123,143)(H,145,146)(H4,95,96,106)(H4,97,98,107)(H4,99,100,108)(H4,101,102,109)(H4,103,104,110)/t55-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Polytechnique F�d�rale de Lausanne (EPFL)
Curated by ChEMBL
| Assay Description
Inhibition of human beta factor 12a using fluorogenic substrate Boc-Gln-Gly-Arg-AMC preincubated for 10 mins followed by addition of substrate measur... |
J Med Chem 60: 1151-1158 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01548
BindingDB Entry DOI: 10.7270/Q26D5W8V |
More data for this
Ligand-Target Pair | |
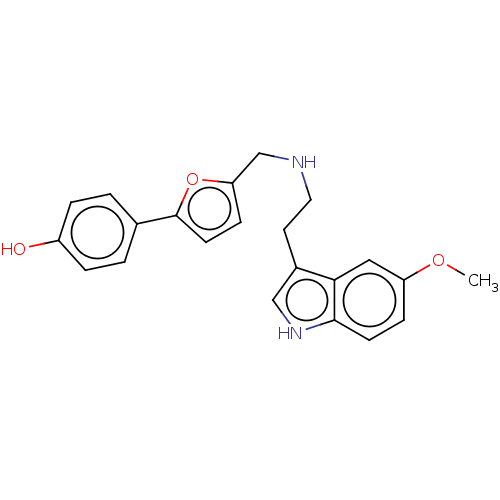
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50504842
(CHEMBL4583082)Show SMILES COc1ccc2[nH]cc(CCNCc3ccc(o3)-c3ccc(O)cc3)c2c1 Show InChI InChI=1S/C22H22N2O3/c1-26-18-6-8-21-20(12-18)16(13-24-21)10-11-23-14-19-7-9-22(27-19)15-2-4-17(25)5-3-15/h2-9,12-13,23-25H,10-11,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6R expressed in HEK293 cells after 1 hr by microbeta plate reader analysis |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50019754
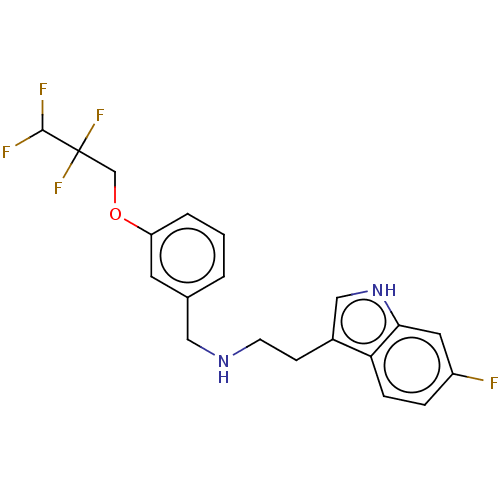
(IDALOPIRDINE | LU-AE58054)Show SMILES FC(F)C(F)(F)COc1cccc(CNCCc2c[nH]c3cc(F)ccc23)c1 Show InChI InChI=1S/C20H19F5N2O/c21-15-4-5-17-14(11-27-18(17)9-15)6-7-26-10-13-2-1-3-16(8-13)28-12-20(24,25)19(22)23/h1-5,8-9,11,19,26-27H,6-7,10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6R expressed in HEK293 cells after 1 hr by microbeta plate reader analysis |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |
Coagulation factor XII
(Homo sapiens (Human)) | BDBM50229997
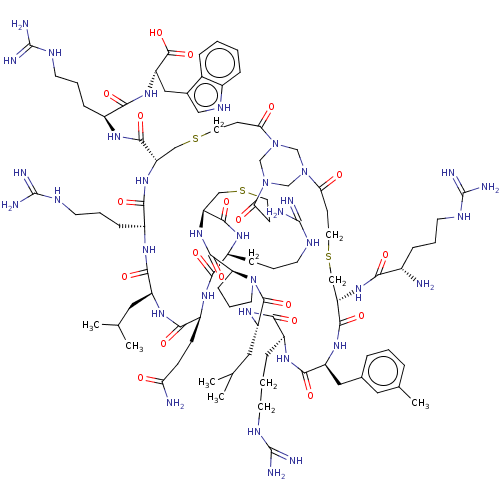
(CHEMBL4065450)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CSCCC(=O)N3CN(CN(C3)C(=O)CCSC[C@H](NC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](Cc3cccc(C)c3)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N3CCC[C@H]3C(=O)N2)C(=O)CCSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O |r| Show InChI InChI=1S/C94H150N34O19S3/c1-51(2)39-64-82(139)113-62(23-13-34-111-94(105)106)80(137)123-69(84(141)116-61(22-12-33-110-93(103)104)79(136)121-67(89(146)147)43-55-44-112-58-19-7-6-17-56(55)58)46-149-37-28-74(131)126-48-125-49-127(50-126)75(132)29-38-150-47-70(85(142)115-59(20-10-31-108-91(99)100)77(134)117-63(81(138)118-64)25-26-72(96)129)124-87(144)71-24-14-35-128(71)88(145)66(40-52(3)4)120-78(135)60(21-11-32-109-92(101)102)114-83(140)65(42-54-16-8-15-53(5)41-54)119-86(143)68(45-148-36-27-73(125)130)122-76(133)57(95)18-9-30-107-90(97)98/h6-8,15-17,19,41,44,51-52,57,59-71,112H,9-14,18,20-40,42-43,45-50,95H2,1-5H3,(H2,96,129)(H,113,139)(H,114,140)(H,115,142)(H,116,141)(H,117,134)(H,118,138)(H,119,143)(H,120,135)(H,121,136)(H,122,133)(H,123,137)(H,124,144)(H,146,147)(H4,97,98,107)(H4,99,100,108)(H4,101,102,109)(H4,103,104,110)(H4,105,106,111)/t57-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Polytechnique F�d�rale de Lausanne (EPFL)
Curated by ChEMBL
| Assay Description
Inhibition of human beta factor 12a using fluorogenic substrate Boc-Gln-Gly-Arg-AMC preincubated for 10 mins followed by addition of substrate measur... |
J Med Chem 60: 1151-1158 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01548
BindingDB Entry DOI: 10.7270/Q26D5W8V |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50219490
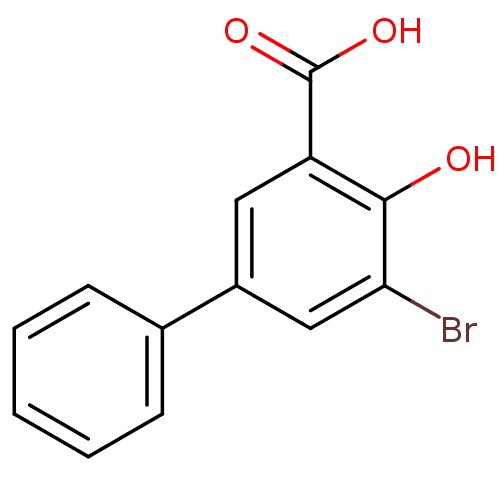
(3-Bromo-5-phenylsalicylc acid | 5-bromo-4-hydroxyb...)Show InChI InChI=1S/C13H9BrO3/c14-11-7-9(8-4-2-1-3-5-8)6-10(12(11)15)13(16)17/h1-7,15H,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 20-alpha HSD expressed in Escherichia coli JM109 |
J Med Chem 52: 3259-64 (2009)
Article DOI: 10.1021/jm9001633
BindingDB Entry DOI: 10.7270/Q2765F6T |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334252
(3-(4-methylpiperazin-1-yl)-1-(phenylsulfonyl)-1H-i...)Show SMILES CN1CCN(CC1)c1cn(c2ccccc12)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C19H21N3O2S/c1-20-11-13-21(14-12-20)19-15-22(18-10-6-5-9-17(18)19)25(23,24)16-7-3-2-4-8-16/h2-10,15H,11-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5-HT6 receptor incubated for 1 hr by microbeta counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112916
BindingDB Entry DOI: 10.7270/Q22R3WD3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50524111

(CHEMBL4452569)Show InChI InChI=1S/C13H11FIN3/c1-2-18-7-16-6-11(18)8-5-17-10-4-3-9(15)13(14)12(8)10/h3-7,17H,2H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human recombinant 5-HT7B receptor expressed in HEK293 cells measured after 1 hr by microbeta scintillation counting me... |
Eur J Med Chem 170: 261-275 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.017
BindingDB Entry DOI: 10.7270/Q2222Z6Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50513435

(CHEMBL4472629)Show SMILES Cl.Cc1nc(N)[nH]c1-c1ccc2ccn(c2c1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C18H16N4O2S/c1-12-17(21-18(19)20-12)14-8-7-13-9-10-22(16(13)11-14)25(23,24)15-5-3-2-4-6-15/h2-11H,1H3,(H3,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells measured after 1 hr by liquid scintillation counter method |
Eur J Med Chem 179: 1-15 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.001
BindingDB Entry DOI: 10.7270/Q2S185TZ |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50219490

(3-Bromo-5-phenylsalicylc acid | 5-bromo-4-hydroxyb...)Show InChI InChI=1S/C13H9BrO3/c14-11-7-9(8-4-2-1-3-5-8)6-10(12(11)15)13(16)17/h1-7,15H,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Coagulation factor XII
(Homo sapiens (Human)) | BDBM50229866
(CHEMBL4069650)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CSCCC(=O)N3CN(CN(C3)C(=O)CCSC[C@H](NC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](Cc3cccc(F)c3)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N3CCC[C@H]3C(=O)N2)C(=O)CCSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O |r| Show InChI InChI=1S/C93H147FN34O19S3/c1-50(2)38-63-81(139)113-61(22-12-33-111-93(105)106)79(137)123-68(83(141)116-60(21-11-32-110-92(103)104)78(136)121-66(88(146)147)42-53-43-112-57-18-6-5-16-55(53)57)45-149-36-27-73(131)126-47-125-48-127(49-126)74(132)28-37-150-46-69(84(142)115-58(19-9-30-108-90(99)100)76(134)117-62(80(138)118-63)24-25-71(96)129)124-86(144)70-23-13-34-128(70)87(145)65(39-51(3)4)120-77(135)59(20-10-31-109-91(101)102)114-82(140)64(41-52-14-7-15-54(94)40-52)119-85(143)67(44-148-35-26-72(125)130)122-75(133)56(95)17-8-29-107-89(97)98/h5-7,14-16,18,40,43,50-51,56,58-70,112H,8-13,17,19-39,41-42,44-49,95H2,1-4H3,(H2,96,129)(H,113,139)(H,114,140)(H,115,142)(H,116,141)(H,117,134)(H,118,138)(H,119,143)(H,120,135)(H,121,136)(H,122,133)(H,123,137)(H,124,144)(H,146,147)(H4,97,98,107)(H4,99,100,108)(H4,101,102,109)(H4,103,104,110)(H4,105,106,111)/t56-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Polytechnique F�d�rale de Lausanne (EPFL)
Curated by ChEMBL
| Assay Description
Inhibition of human beta factor 12a using fluorogenic substrate Boc-Gln-Gly-Arg-AMC preincubated for 10 mins followed by addition of substrate measur... |
J Med Chem 60: 1151-1158 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01548
BindingDB Entry DOI: 10.7270/Q26D5W8V |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330427

(5-Chloro-4-hydroxybiphenyl-3-carboxylic acid | CHE...)Show InChI InChI=1S/C13H9ClO3/c14-11-7-9(8-4-2-1-3-5-8)6-10(12(11)15)13(16)17/h1-7,15H,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant AKR1C1 Leu54Val mutant by fluorescence assay |
Bioorg Med Chem Lett 21: 2564-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.076
BindingDB Entry DOI: 10.7270/Q20G3NZ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50578573

(CHEMBL4869180) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]citalopram from human SERT in HEK293 cells by Topcount scintillation analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113533
BindingDB Entry DOI: 10.7270/Q24T6P6C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50524116
(CHEMBL4469847)Show InChI InChI=1S/C14H14N4O/c1-2-18-8-16-7-13(18)11-6-17-12-4-3-9(14(15)19)5-10(11)12/h3-8,17H,2H2,1H3,(H2,15,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human recombinant 5-HT7B receptor expressed in HEK293 cells measured after 1 hr by microbeta scintillation counting me... |
Eur J Med Chem 170: 261-275 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.017
BindingDB Entry DOI: 10.7270/Q2222Z6Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50504843

(CHEMBL4438434)Show SMILES Cc1ccc2[nH]cc(CCNCc3ccc(o3)-c3ccc(O)cc3)c2c1 Show InChI InChI=1S/C22H22N2O2/c1-15-2-8-21-20(12-15)17(13-24-21)10-11-23-14-19-7-9-22(26-19)16-3-5-18(25)6-4-16/h2-9,12-13,23-25H,10-11,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6R expressed in HEK293 cells after 1 hr by microbeta plate reader analysis |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111857
BindingDB Entry DOI: 10.7270/Q2833W92 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHOK1 cells incubated for 1 hr by liquid scintillation counter method |
Eur J Med Chem 179: 1-15 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.001
BindingDB Entry DOI: 10.7270/Q2S185TZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H] ketanserin from human recombinant 5-HT2A receptor expressed in CHOK1 cells after 1 hr by microbeta scintillation counting method |
Eur J Med Chem 170: 261-275 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.017
BindingDB Entry DOI: 10.7270/Q2222Z6Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHOK1 cells incubated for 1 hr by liquid scintillation counter method |
Eur J Med Chem 179: 1-15 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.001
BindingDB Entry DOI: 10.7270/Q2S185TZ |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM26269
(3,5-dichloro-2-hydroxybenzoic acid | 3,5-dichloros...)Show InChI InChI=1S/C7H4Cl2O3/c8-3-1-4(7(11)12)6(10)5(9)2-3/h1-2,10H,(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.90 | -47.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Monash University
| Assay Description
The activity was assayed by measuring the rate of change in NADPH fluorescence (at 455 nm with an excitation wavelength of 340 nm) at 298 K. When the... |
J Med Chem 51: 4844-8 (2008)
Article DOI: 10.1021/jm8003575
BindingDB Entry DOI: 10.7270/Q2MG7MTW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM26269

(3,5-dichloro-2-hydroxybenzoic acid | 3,5-dichloros...)Show InChI InChI=1S/C7H4Cl2O3/c8-3-1-4(7(11)12)6(10)5(9)2-3/h1-2,10H,(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data