| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Nitric oxide synthase, endothelial |
|---|
| Ligand | BDBM50403161 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1738191 (CHEMBL4153941) |
|---|
| IC50 | >1000000±n/a nM |
|---|
| Citation |  Maccallini, C; Di Matteo, M; Gallorini, M; Montagnani, M; Graziani, V; Ammazzalorso, A; Amoia, P; De Filippis, B; Di Silvestre, S; Fantacuzzi, M; Giampietro, L; Potenza, MA; Re, N; Pandolfi, A; Cataldi, A; Amoroso, R Discovery of N-{3-[(ethanimidoylamino)methyl]benzyl}-l-prolinamide dihydrochloride: A new potent and selective inhibitor of the inducible nitric oxide synthase as a promising agent for the therapy of malignant glioma. Eur J Med Chem152:53-64 (2018) [PubMed] Article Maccallini, C; Di Matteo, M; Gallorini, M; Montagnani, M; Graziani, V; Ammazzalorso, A; Amoia, P; De Filippis, B; Di Silvestre, S; Fantacuzzi, M; Giampietro, L; Potenza, MA; Re, N; Pandolfi, A; Cataldi, A; Amoroso, R Discovery of N-{3-[(ethanimidoylamino)methyl]benzyl}-l-prolinamide dihydrochloride: A new potent and selective inhibitor of the inducible nitric oxide synthase as a promising agent for the therapy of malignant glioma. Eur J Med Chem152:53-64 (2018) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Nitric oxide synthase, endothelial |
|---|
| Name: | Nitric oxide synthase, endothelial |
|---|
| Synonyms: | Constitutive NOS | Endothelial NOS | Endothelial nitric oxide synthase | Endothelial nitric-oxide synthase (eNOS) | NOS type III | NOS3 | NOS3_BOVIN | Nitric oxide synthase, endothelial (eNOS) | Nitric-oxide synthase, endothelial | cNOS |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 133292.26 |
|---|
| Organism: | Bos taurus (bovine) |
|---|
| Description: | Recombinant eNOS overexpressed in E. coli was used in enzyme assays. |
|---|
| Residue: | 1205 |
|---|
| Sequence: | MGNLKSVGQEPGPPCGLGLGLGLGLCGKQGPASPAPEPSRAPAPATPHAPDHSPAPNSPT
LTRPPEGPKFPRVKNWELGSITYDTLCAQSQQDGPCTPRCCLGSLVLPRKLQTRPSPGPP
PAEQLLSQARDFINQYYSSIKRSGSQAHEERLQEVEAEVASTGTYHLRESELVFGAKQAW
RNAPRCVGRIQWGKLQVFDARDCSSAQEMFTYICNHIKYATNRGNLRSAITVFPQRAPGR
GDFRIWNSQLVRYAGYRQQDGSVRGDPANVEITELCIQHGWTPGNGRFDVLPLLLQAPDE
APELFVLPPELVLEVPLEHPTLEWFAALGLRWYALPAVSNMLLEIGGLEFSAAPFSGWYM
STEIGTRNLCDPHRYNILEDVAVCMDLDTRTTSSLWKDKAAVEINLAVLHSFQLAKVTIV
DHHAATVSFMKHLDNEQKARGGCPADWAWIVPPISGSLTPVFHQEMVNYILSPAFRYQPD
PWKGSATKGAGITRKKTFKEVANAVKISASLMGTLMAKRVKATILYASETGRAQSYAQQL
GRLFRKAFDPRVLCMDEYDVVSLEHEALVLVVTSTFGNGDPPENGESFAAALMEMSGPYN
SSPRPEQHKSYKIRFNSVSCSDPLVSSWRRKRKESSNTDSAGALGTLRFCVFGLGSRAYP
HFCAFARAVDTRLEELGGERLLQLGQGDELCGQEEAFRGWAKAAFQASCETFCVGEEAKA
AAQDIFSPKRSWKRQRYRLSTQAEGLQLLPGLIHVHRRKMFQATVLSVENLQSSKSTRAT
ILVRLDTAGQEGLQYQPGDHIGICPPNRPGLVEALLSRVEDPPPPTESVAVEQLEKGSPG
GPPPSWVRDPRLPPCTLRQALTFFLDITSPPSPRLLRLLSTLAEEPSEQQELETLSQDPR
RYEEWKWFRCPTLLEVLEQFPSVALPAPLLLTQLPLLQPRYYSVSSAPNAHPGEVHLTVA
VLAYRTQDGLGPLHYGVCSTWLSQLKTGDPVPCFIRGAPSFRLPPDPYVPCILVGPGTGI
APFRGFWQERLHDIESKGLQPAPMTLVFGCRCSQLDHLYRDEVQDAQERGVFGRVLTAFS
REPDSPKTYVQDILRTELAAEVHRVLCLERGHMFVCGDVTMATSVLQTVQRILATEGDME
LDEAGDVIGVLRDQQRYHEDIFGLTLRTQEVTSRIRTQSFSLQERHLRGAVPWAFDPPGP
DTPGP
|
|
|
|---|
| BDBM50403161 |
|---|
| n/a |
|---|
| Name | BDBM50403161 |
|---|
| Synonyms: | CHEMBL4177347 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H30Cl3N7O |
|---|
| Mol. Mass. | 442.815 |
|---|
| SMILES | Cl.Cl.Cl.CC(=N)NCc1cccc(CNC(=O)[C@@H](N)CCCNC(N)=N)c1 |r| |
|---|
| Structure | 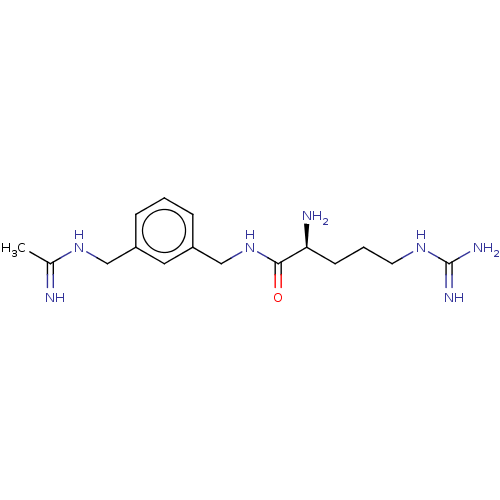
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Maccallini, C; Di Matteo, M; Gallorini, M; Montagnani, M; Graziani, V; Ammazzalorso, A; Amoia, P; De Filippis, B; Di Silvestre, S; Fantacuzzi, M; Giampietro, L; Potenza, MA; Re, N; Pandolfi, A; Cataldi, A; Amoroso, R Discovery of N-{3-[(ethanimidoylamino)methyl]benzyl}-l-prolinamide dihydrochloride: A new potent and selective inhibitor of the inducible nitric oxide synthase as a promising agent for the therapy of malignant glioma. Eur J Med Chem152:53-64 (2018) [PubMed] Article
Maccallini, C; Di Matteo, M; Gallorini, M; Montagnani, M; Graziani, V; Ammazzalorso, A; Amoia, P; De Filippis, B; Di Silvestre, S; Fantacuzzi, M; Giampietro, L; Potenza, MA; Re, N; Pandolfi, A; Cataldi, A; Amoroso, R Discovery of N-{3-[(ethanimidoylamino)methyl]benzyl}-l-prolinamide dihydrochloride: A new potent and selective inhibitor of the inducible nitric oxide synthase as a promising agent for the therapy of malignant glioma. Eur J Med Chem152:53-64 (2018) [PubMed] Article