

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50036738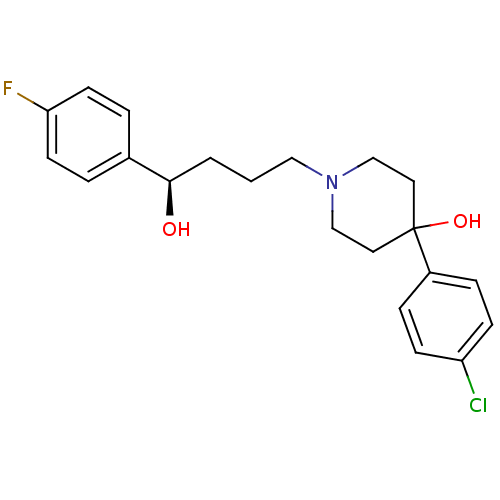 ((R)-4-(4-Chloro-phenyl)-1-[4-(4-fluoro-phenyl)-4-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor guinea pig brain membranes incubated for 150 mins by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine D2 receptor in rat striatum by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor guinea pig brain membranes incubated for 150 mins by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50036734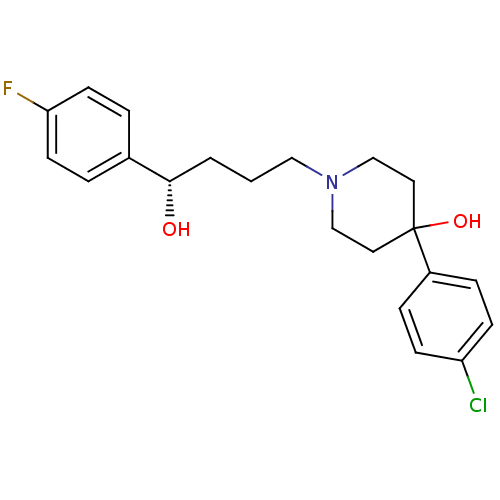 ((S)-4-(4-Chloro-phenyl)-1-[4-(4-fluoro-phenyl)-4-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor guinea pig brain membranes incubated for 150 mins by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-7-OH-DPAT from dopamine D3 receptor in rat olfactory tubercle by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50009307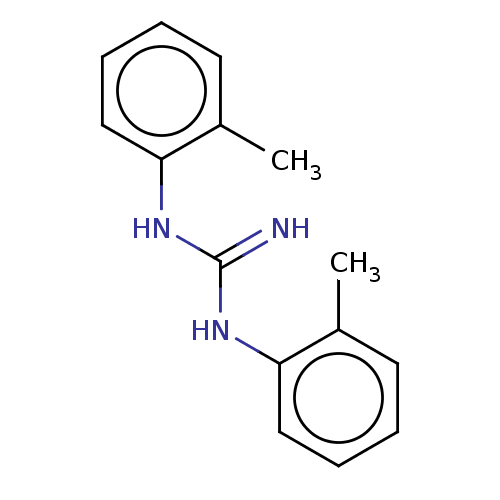 (DITOLYLGUANIDINE | Di-o-tolylguanidine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor guinea pig brain membranes incubated for 150 mins by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50036734 ((S)-4-(4-Chloro-phenyl)-1-[4-(4-fluoro-phenyl)-4-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine D2 receptor in rat striatum by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50059789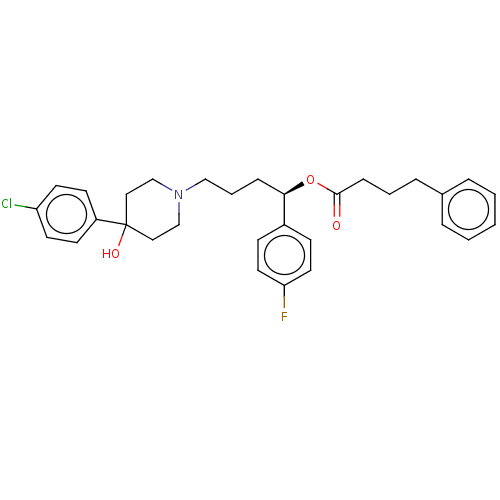 (CHEMBL3393789) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor guinea pig brain membranes incubated for 150 mins by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50059787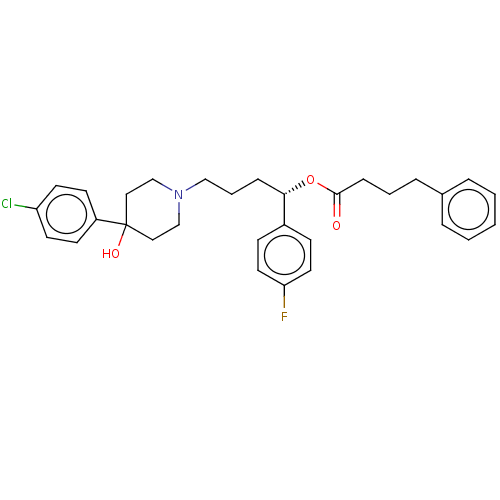 (CHEMBL3393790) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor guinea pig brain membranes incubated for 150 mins by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50036738 ((R)-4-(4-Chloro-phenyl)-1-[4-(4-fluoro-phenyl)-4-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 256 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine D2 receptor in rat striatum by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50036734 ((S)-4-(4-Chloro-phenyl)-1-[4-(4-fluoro-phenyl)-4-h...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 353 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-7-OH-DPAT from dopamine D3 receptor in rat olfactory tubercle by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50036738 ((R)-4-(4-Chloro-phenyl)-1-[4-(4-fluoro-phenyl)-4-h...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-7-OH-DPAT from dopamine D3 receptor in rat olfactory tubercle by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50059787 (CHEMBL3393790) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine D2 receptor in rat striatum by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50059789 (CHEMBL3393789) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-7-OH-DPAT from dopamine D3 receptor in rat olfactory tubercle by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50059787 (CHEMBL3393790) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-7-OH-DPAT from dopamine D3 receptor in rat olfactory tubercle by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50059789 (CHEMBL3393789) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Chieti Gabriele D'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine D2 receptor in rat striatum by scintillation counting method | Eur J Med Chem 90: 1-9 (2015) Article DOI: 10.1016/j.ejmech.2014.11.012 BindingDB Entry DOI: 10.7270/Q2WS8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM13061 (4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. d'Annunzio" University Curated by ChEMBL | Assay Description Inhibition of aromatase in human MCF-7aro cells using [1beta-3H] androstenedione as substrate incubated for 1 hr by liquid scintillation counting met... | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111815 BindingDB Entry DOI: 10.7270/Q2NC64GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50543107 (CHEMBL4633346) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University "G. d'Annunzio" of Chieti-Pescara Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human iNOS expressed in Escherichia coli using L-arginine as substrate preincubated for 15 mins follo... | ACS Med Chem Lett 11: 1470-1475 (2020) Article DOI: 10.1021/acsmedchemlett.0c00285 BindingDB Entry DOI: 10.7270/Q2X06BMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50401339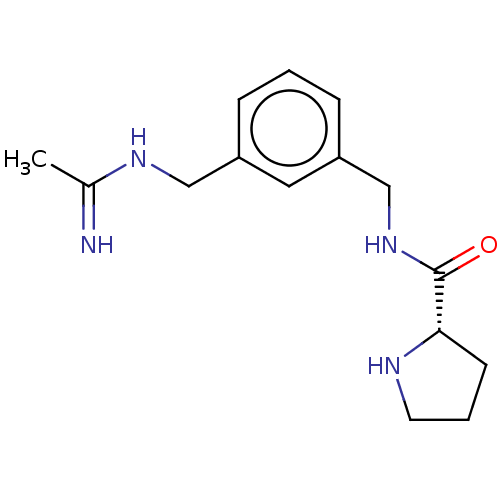 (CHEMBL4161447) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti "G. d'Annunzio" Curated by ChEMBL | Assay Description Inhibition of recombinant mouse iNOS using [3H]L-arginine as substrate preincubated for 15 mins followed by NADPH addition measured after 20 mins in ... | Eur J Med Chem 152: 53-64 (2018) Article DOI: 10.1016/j.ejmech.2018.04.027 BindingDB Entry DOI: 10.7270/Q2C2500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50226527 (CHEMBL107251 | N-(3-(AMINOMETHYL)BENZYL)ACETAMIDIN...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University "G. d'Annunzio" of Chieti-Pescara Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human iNOS expressed in Escherichia coli using L-arginine as substrate preincubated for 15 mins follo... | ACS Med Chem Lett 11: 1470-1475 (2020) Article DOI: 10.1021/acsmedchemlett.0c00285 BindingDB Entry DOI: 10.7270/Q2X06BMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50226527 (CHEMBL107251 | N-(3-(AMINOMETHYL)BENZYL)ACETAMIDIN...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti "G. d'Annunzio" Curated by ChEMBL | Assay Description Inhibition of recombinant mouse iNOS using [3H]L-arginine as substrate preincubated for 15 mins followed by NADPH addition measured after 20 mins in ... | Eur J Med Chem 152: 53-64 (2018) Article DOI: 10.1016/j.ejmech.2018.04.027 BindingDB Entry DOI: 10.7270/Q2C2500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50504788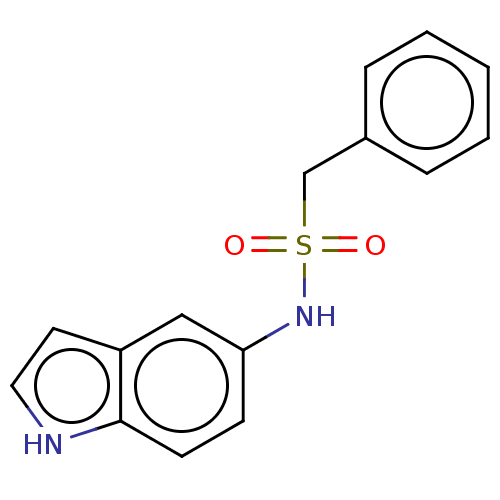 (CHEMBL4537791) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. d'Annunzio" University Curated by ChEMBL | Assay Description Inhibition of recombinant human aromatase preincubated for 10 mins followed by substrate and beta-NADP+ addition and measured for 60 mins by fluorime... | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111815 BindingDB Entry DOI: 10.7270/Q2NC64GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50403157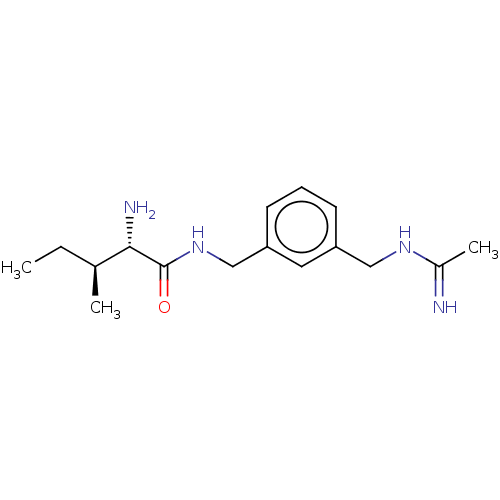 (CHEMBL4171271) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti "G. d'Annunzio" Curated by ChEMBL | Assay Description Inhibition of recombinant mouse iNOS using [3H]L-arginine as substrate preincubated for 15 mins followed by NADPH addition measured after 20 mins in ... | Eur J Med Chem 152: 53-64 (2018) Article DOI: 10.1016/j.ejmech.2018.04.027 BindingDB Entry DOI: 10.7270/Q2C2500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50504787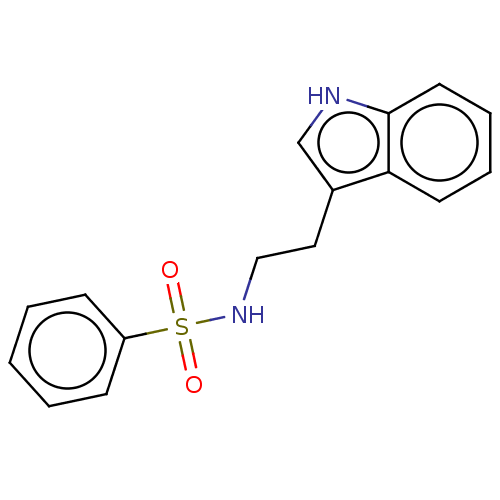 (CHEMBL1371590) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. d'Annunzio" University Curated by ChEMBL | Assay Description Inhibition of recombinant human aromatase preincubated for 10 mins followed by substrate and beta-NADP+ addition and measured for 60 mins by fluorime... | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111815 BindingDB Entry DOI: 10.7270/Q2NC64GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50403472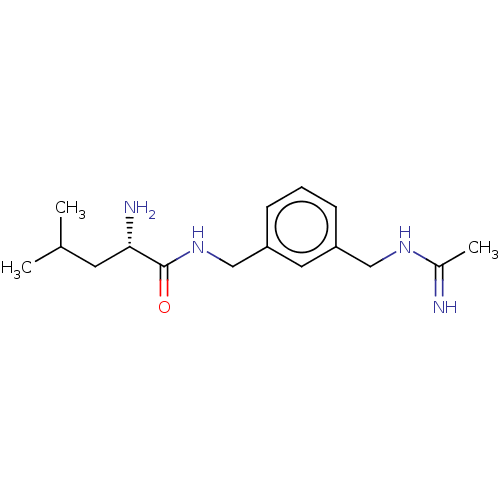 (CHEMBL4169004) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 253 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti "G. d'Annunzio" Curated by ChEMBL | Assay Description Inhibition of recombinant mouse iNOS using [3H]L-arginine as substrate preincubated for 15 mins followed by NADPH addition measured after 20 mins in ... | Eur J Med Chem 152: 53-64 (2018) Article DOI: 10.1016/j.ejmech.2018.04.027 BindingDB Entry DOI: 10.7270/Q2C2500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50403162 (CHEMBL4160671) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 276 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti "G. d'Annunzio" Curated by ChEMBL | Assay Description Inhibition of recombinant mouse iNOS using [3H]L-arginine as substrate preincubated for 15 mins followed by NADPH addition measured after 20 mins in ... | Eur J Med Chem 152: 53-64 (2018) Article DOI: 10.1016/j.ejmech.2018.04.027 BindingDB Entry DOI: 10.7270/Q2C2500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50095298 (CHEMBL3589087) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 428 | n/a | n/a | n/a | n/a | n/a | n/a |
University "G. d'Annunzio" of Chieti-Pescara Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human iNOS expressed in Escherichia coli using L-arginine as substrate preincubated for 15 mins follo... | ACS Med Chem Lett 11: 1470-1475 (2020) Article DOI: 10.1021/acsmedchemlett.0c00285 BindingDB Entry DOI: 10.7270/Q2X06BMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50403160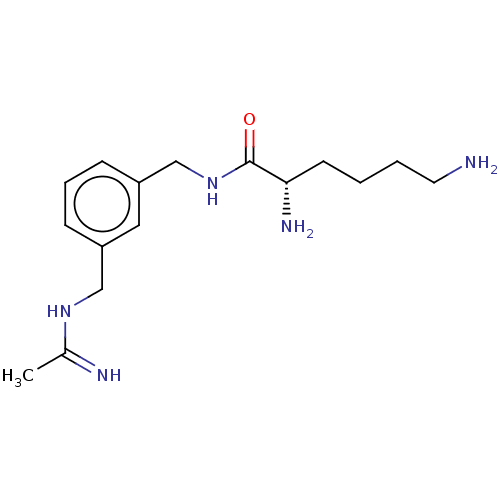 (CHEMBL4169649) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 446 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti "G. d'Annunzio" Curated by ChEMBL | Assay Description Inhibition of recombinant mouse iNOS using [3H]L-arginine as substrate preincubated for 15 mins followed by NADPH addition measured after 20 mins in ... | Eur J Med Chem 152: 53-64 (2018) Article DOI: 10.1016/j.ejmech.2018.04.027 BindingDB Entry DOI: 10.7270/Q2C2500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50403161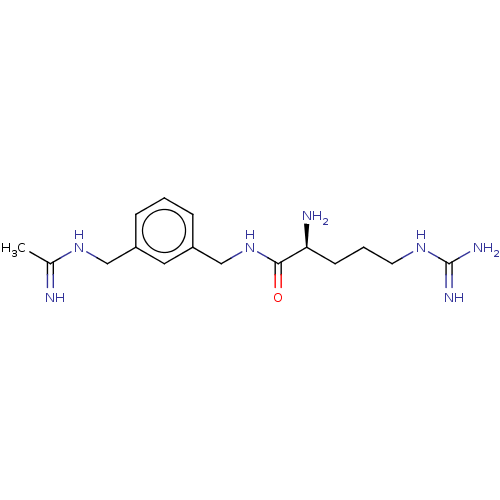 (CHEMBL4177347) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti "G. d'Annunzio" Curated by ChEMBL | Assay Description Inhibition of recombinant mouse iNOS using [3H]L-arginine as substrate preincubated for 15 mins followed by NADPH addition measured after 20 mins in ... | Eur J Med Chem 152: 53-64 (2018) Article DOI: 10.1016/j.ejmech.2018.04.027 BindingDB Entry DOI: 10.7270/Q2C2500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50504786 (CHEMBL4516755) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. d'Annunzio" University Curated by ChEMBL | Assay Description Inhibition of recombinant human aromatase preincubated for 10 mins followed by substrate and beta-NADP+ addition and measured for 60 mins by fluorime... | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111815 BindingDB Entry DOI: 10.7270/Q2NC64GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50403159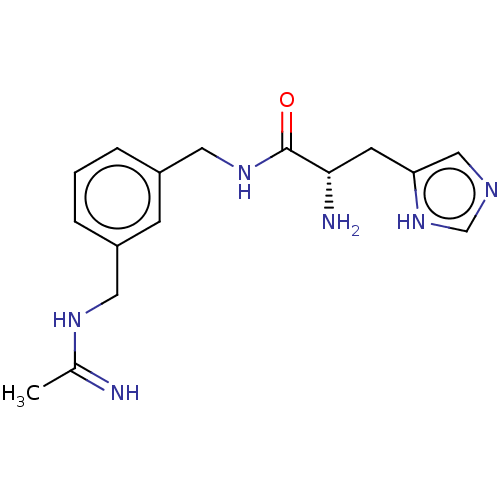 (CHEMBL4173076) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 534 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti "G. d'Annunzio" Curated by ChEMBL | Assay Description Inhibition of recombinant mouse iNOS using [3H]L-arginine as substrate preincubated for 15 mins followed by NADPH addition measured after 20 mins in ... | Eur J Med Chem 152: 53-64 (2018) Article DOI: 10.1016/j.ejmech.2018.04.027 BindingDB Entry DOI: 10.7270/Q2C2500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50543108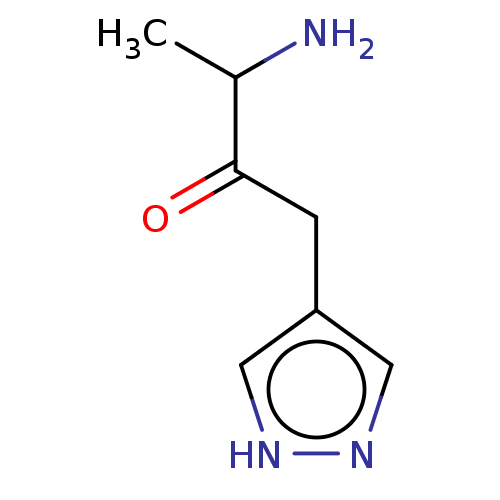 (CHEMBL4635568) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 681 | n/a | n/a | n/a | n/a | n/a | n/a |
University "G. d'Annunzio" of Chieti-Pescara Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human iNOS expressed in Escherichia coli using L-arginine as substrate preincubated for 15 mins follo... | ACS Med Chem Lett 11: 1470-1475 (2020) Article DOI: 10.1021/acsmedchemlett.0c00285 BindingDB Entry DOI: 10.7270/Q2X06BMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50504789 (CHEMBL4464506) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. d'Annunzio" University Curated by ChEMBL | Assay Description Inhibition of recombinant human aromatase preincubated for 10 mins followed by substrate and beta-NADP+ addition and measured for 60 mins by fluorime... | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111815 BindingDB Entry DOI: 10.7270/Q2NC64GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50570215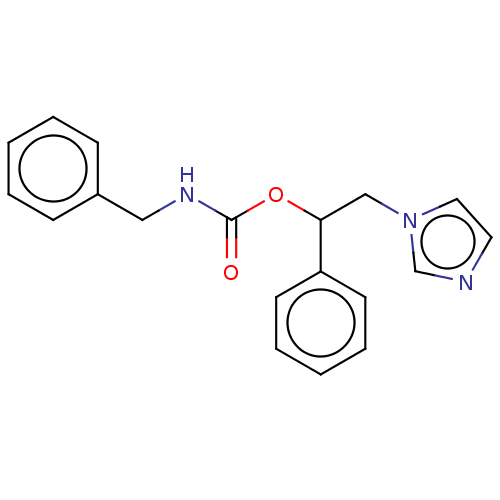 (CHEMBL4858552) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CYP19A measured every 1 min for 60 mins by flourimetric assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113115 BindingDB Entry DOI: 10.7270/Q2XP78PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50570216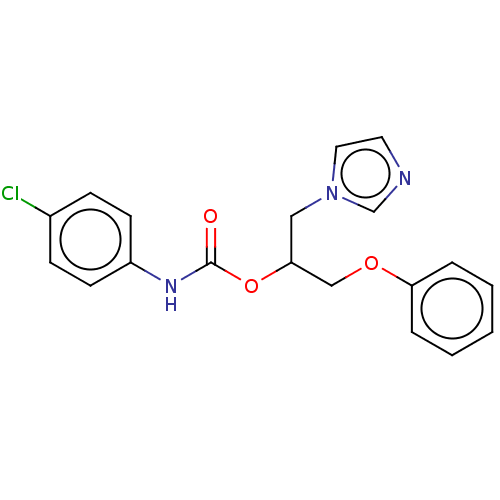 (CHEMBL4874326) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CYP19A measured every 1 min for 60 mins by flourimetric assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113115 BindingDB Entry DOI: 10.7270/Q2XP78PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50403156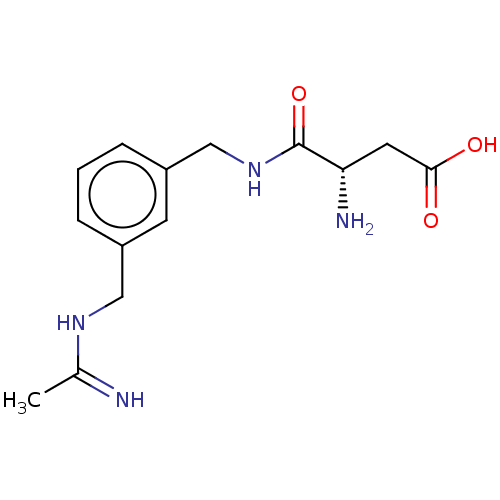 (CHEMBL4176848) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti "G. d'Annunzio" Curated by ChEMBL | Assay Description Inhibition of recombinant mouse iNOS using [3H]L-arginine as substrate preincubated for 15 mins followed by NADPH addition measured after 20 mins in ... | Eur J Med Chem 152: 53-64 (2018) Article DOI: 10.1016/j.ejmech.2018.04.027 BindingDB Entry DOI: 10.7270/Q2C2500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50403464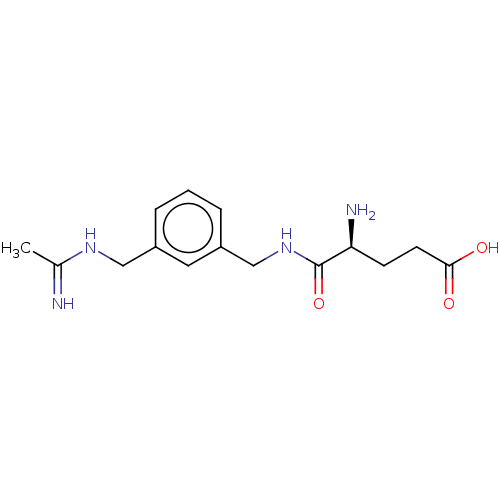 (CHEMBL4166234) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti "G. d'Annunzio" Curated by ChEMBL | Assay Description Inhibition of recombinant mouse iNOS using [3H]L-arginine as substrate preincubated for 15 mins followed by NADPH addition measured after 20 mins in ... | Eur J Med Chem 152: 53-64 (2018) Article DOI: 10.1016/j.ejmech.2018.04.027 BindingDB Entry DOI: 10.7270/Q2C2500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50573759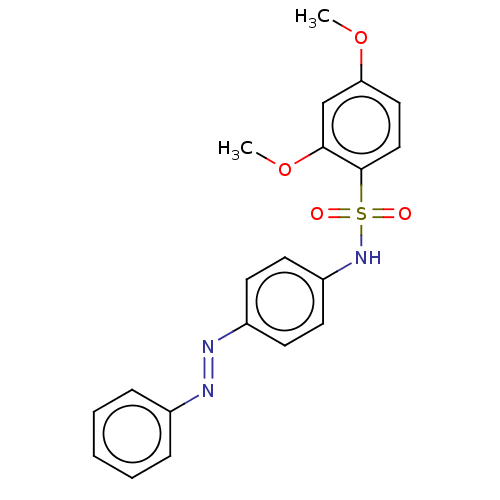 (CHEMBL4870604) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human aromatase assessed as reduction in fluorescence intensity using 7-methoxy-4-trifluoromethyl coumarin as a substrate by fluorimetr... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113737 BindingDB Entry DOI: 10.7270/Q20G3PZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50573760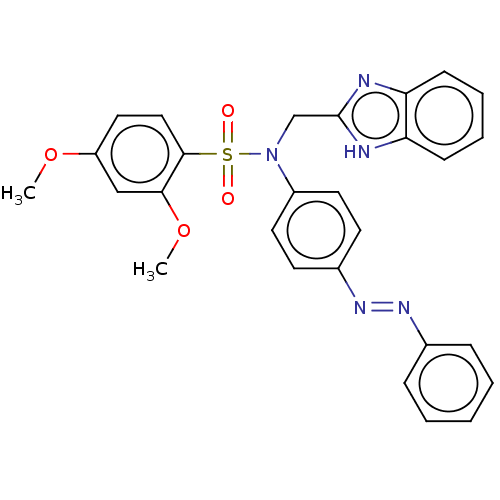 (CHEMBL4868867) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human aromatase assessed as reduction in fluorescence intensity using 7-methoxy-4-trifluoromethyl coumarin as a substrate by fluorimetr... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113737 BindingDB Entry DOI: 10.7270/Q20G3PZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50210930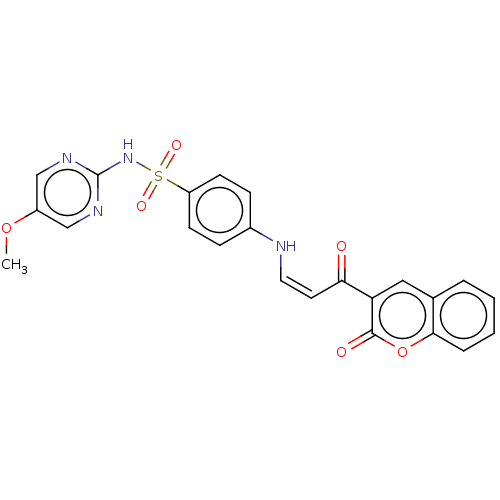 (CHEMBL3924170) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human aromatase in T47D measured after 24 hrs by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113737 BindingDB Entry DOI: 10.7270/Q20G3PZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50543107 (CHEMBL4633346) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University "G. d'Annunzio" of Chieti-Pescara Curated by ChEMBL | Assay Description Inhibition of recombinant human nNOS expressed in Escherichia coli using L-arginine as substrate preincubated for 15 mins followed by NADPH addition ... | ACS Med Chem Lett 11: 1470-1475 (2020) Article DOI: 10.1021/acsmedchemlett.0c00285 BindingDB Entry DOI: 10.7270/Q2X06BMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50226527 (CHEMBL107251 | N-(3-(AMINOMETHYL)BENZYL)ACETAMIDIN...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti "G. d'Annunzio" Curated by ChEMBL | Assay Description Inhibition of recombinant rat nNOS using [3H]L-arginine as substrate preincubated for 15 mins followed by NADPH addition measured after 20 mins in pr... | Eur J Med Chem 152: 53-64 (2018) Article DOI: 10.1016/j.ejmech.2018.04.027 BindingDB Entry DOI: 10.7270/Q2C2500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50226527 (CHEMBL107251 | N-(3-(AMINOMETHYL)BENZYL)ACETAMIDIN...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University "G. d'Annunzio" of Chieti-Pescara Curated by ChEMBL | Assay Description Inhibition of recombinant human nNOS expressed in Escherichia coli using L-arginine as substrate preincubated for 15 mins followed by NADPH addition ... | ACS Med Chem Lett 11: 1470-1475 (2020) Article DOI: 10.1021/acsmedchemlett.0c00285 BindingDB Entry DOI: 10.7270/Q2X06BMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50573758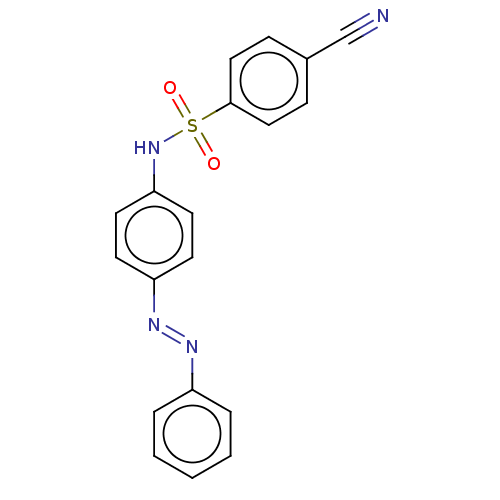 (CHEMBL4847316) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human aromatase assessed as reduction in fluorescence intensity using 7-methoxy-4-trifluoromethyl coumarin as a substrate by fluorimetr... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113737 BindingDB Entry DOI: 10.7270/Q20G3PZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50345657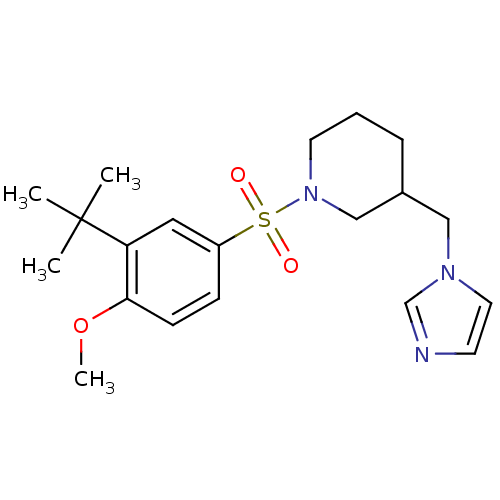 (CHEMBL1784801 | rac-3-((1H-imidazol-1-yl)methyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human aromatase assessed as reduction in fluorescence intensity using 7-methoxy-4-trifluoromethyl coumarin as a substrate by fluorimetr... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113737 BindingDB Entry DOI: 10.7270/Q20G3PZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50403464 (CHEMBL4166234) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti "G. d'Annunzio" Curated by ChEMBL | Assay Description Inhibition of recombinant bovine eNOS using [3H]L-arginine as substrate preincubated for 15 mins followed by NADPH addition measured after 20 mins in... | Eur J Med Chem 152: 53-64 (2018) Article DOI: 10.1016/j.ejmech.2018.04.027 BindingDB Entry DOI: 10.7270/Q2C2500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50543107 (CHEMBL4633346) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University "G. d'Annunzio" of Chieti-Pescara Curated by ChEMBL | Assay Description Inhibition of human eNOS using L-argininge as substrate preincubated for 15 mins followed by NADPH addition and measured after 20 mins by OPA/NAC rea... | ACS Med Chem Lett 11: 1470-1475 (2020) Article DOI: 10.1021/acsmedchemlett.0c00285 BindingDB Entry DOI: 10.7270/Q2X06BMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50403158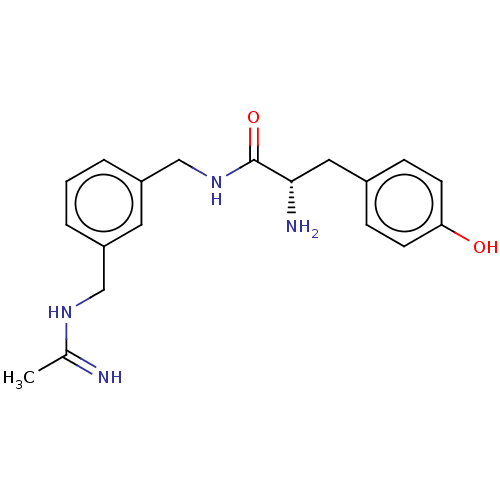 (CHEMBL4163633) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti "G. d'Annunzio" Curated by ChEMBL | Assay Description Inhibition of recombinant mouse iNOS using [3H]L-arginine as substrate preincubated for 15 mins followed by NADPH addition measured after 20 mins in ... | Eur J Med Chem 152: 53-64 (2018) Article DOI: 10.1016/j.ejmech.2018.04.027 BindingDB Entry DOI: 10.7270/Q2C2500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50400786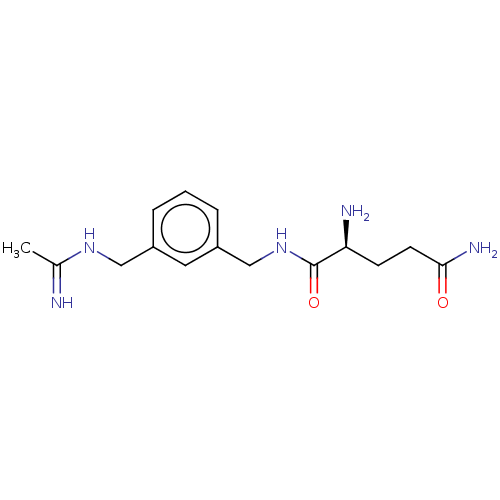 (CHEMBL4171037) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti "G. d'Annunzio" Curated by ChEMBL | Assay Description Inhibition of recombinant mouse iNOS using [3H]L-arginine as substrate preincubated for 15 mins followed by NADPH addition measured after 20 mins in ... | Eur J Med Chem 152: 53-64 (2018) Article DOI: 10.1016/j.ejmech.2018.04.027 BindingDB Entry DOI: 10.7270/Q2C2500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50403471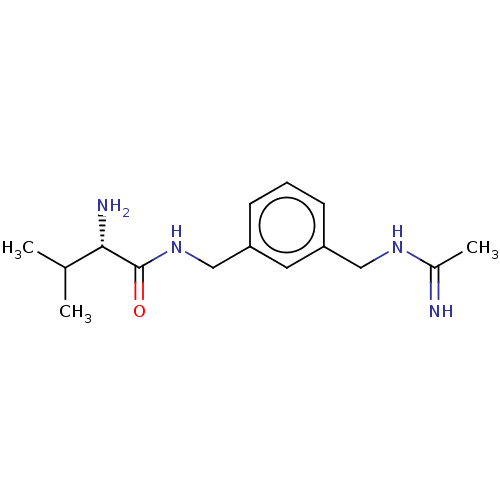 (CHEMBL4161080) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti "G. d'Annunzio" Curated by ChEMBL | Assay Description Inhibition of recombinant mouse iNOS using [3H]L-arginine as substrate preincubated for 15 mins followed by NADPH addition measured after 20 mins in ... | Eur J Med Chem 152: 53-64 (2018) Article DOI: 10.1016/j.ejmech.2018.04.027 BindingDB Entry DOI: 10.7270/Q2C2500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 70 total ) | Next | Last >> |