| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Gastrin/cholecystokinin type B receptor |
|---|
| Ligand | BDBM50454482 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_47830 (CHEMBL662572) |
|---|
| IC50 | 0.280000±n/a nM |
|---|
| Citation |  Castro, JL; Ball, RG; Broughton, HB; Russell, MG; Rathbone, D; Watt, AP; Baker, R; Chapman, KL; Fletcher, AE; Patel, S; Smith, AJ; Marshall, GR; Ryecroft, W; Matassa, VG Controlled modification of acidity in cholecystokinin B receptor antagonists: N-(1,4-benzodiazepin-3-yl)-N'-[3-(tetrazol-5-ylamino) phenyl]ureas. J Med Chem39:842-9 (1996) [PubMed] Article Castro, JL; Ball, RG; Broughton, HB; Russell, MG; Rathbone, D; Watt, AP; Baker, R; Chapman, KL; Fletcher, AE; Patel, S; Smith, AJ; Marshall, GR; Ryecroft, W; Matassa, VG Controlled modification of acidity in cholecystokinin B receptor antagonists: N-(1,4-benzodiazepin-3-yl)-N'-[3-(tetrazol-5-ylamino) phenyl]ureas. J Med Chem39:842-9 (1996) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Gastrin/cholecystokinin type B receptor |
|---|
| Name: | Gastrin/cholecystokinin type B receptor |
|---|
| Synonyms: | CCK-2 receptor | CCK-B receptor | CCK-BR | CCKBR | CCKRB | Cholecystokinin A | Cholecystokinin receptor | Cholecystokinin-2 Receptor | GASR_HUMAN | Gastrin/cholecystokinin type B receptor |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 48445.79 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Stable expression of human CCK-2 receptors in HEK 293 cells. |
|---|
| Residue: | 447 |
|---|
| Sequence: | MELLKLNRSVQGTGPGPGASLCRPGAPLLNSSSVGNLSCEPPRIRGAGTRELELAIRITL
YAVIFLMSVGGNMLIIVVLGLSRRLRTVTNAFLLSLAVSDLLLAVACMPFTLLPNLMGTF
IFGTVICKAVSYLMGVSVSVSTLSLVAIALERYSAICRPLQARVWQTRSHAARVIVATWL
LSGLLMVPYPVYTVVQPVGPRVLQCVHRWPSARVRQTWSVLLLLLLFFIPGVVMAVAYGL
ISRELYLGLRFDGDSDSDSQSRVRNQGGLPGAVHQNGRCRPETGAVGEDSDGCYVQLPRS
RPALELTALTAPGPGSGSRPTQAKLLAKKRVVRMLLVIVVLFFLCWLPVYSANTWRAFDG
PGAHRALSGAPISFIHLLSYASACVNPLVYCFMHRRFRQACLETCARCCPRPPRARPRAL
PDEDPPTPSIASLSRLSYTTISTLGPG
|
|
|
|---|
| BDBM50454482 |
|---|
| n/a |
|---|
| Name | BDBM50454482 |
|---|
| Synonyms: | CHEMBL2110202 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H29N9O2 |
|---|
| Mol. Mass. | 487.5569 |
|---|
| SMILES | CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(CNc3nn[nH]n3)c2)C1=O)C1CCCCC1 |c:9| |
|---|
| Structure | 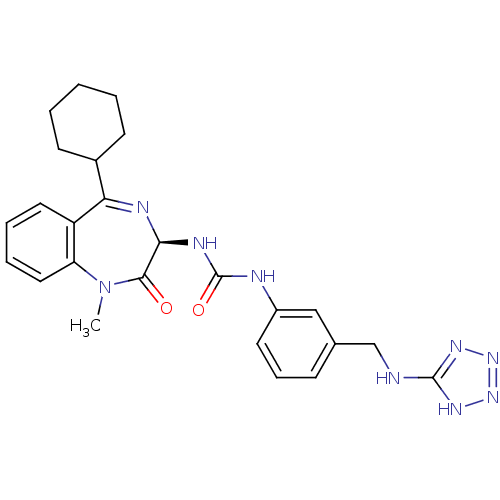
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Castro, JL; Ball, RG; Broughton, HB; Russell, MG; Rathbone, D; Watt, AP; Baker, R; Chapman, KL; Fletcher, AE; Patel, S; Smith, AJ; Marshall, GR; Ryecroft, W; Matassa, VG Controlled modification of acidity in cholecystokinin B receptor antagonists: N-(1,4-benzodiazepin-3-yl)-N'-[3-(tetrazol-5-ylamino) phenyl]ureas. J Med Chem39:842-9 (1996) [PubMed] Article
Castro, JL; Ball, RG; Broughton, HB; Russell, MG; Rathbone, D; Watt, AP; Baker, R; Chapman, KL; Fletcher, AE; Patel, S; Smith, AJ; Marshall, GR; Ryecroft, W; Matassa, VG Controlled modification of acidity in cholecystokinin B receptor antagonists: N-(1,4-benzodiazepin-3-yl)-N'-[3-(tetrazol-5-ylamino) phenyl]ureas. J Med Chem39:842-9 (1996) [PubMed] Article