| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Reverse transcriptase/RNaseH |
|---|
| Ligand | BDBM50521979 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1885886 (CHEMBL4387468) |
|---|
| IC50 | 2000±n/a nM |
|---|
| Citation |  Gao, P; Cheng, X; Sun, L; Song, S; Álvarez, M; Luczkowiak, J; Pannecouque, C; De Clercq, E; Menéndez-Arias, L; Zhan, P; Liu, X Design, synthesis and biological evaluation of 3-hydroxyquinazoline-2,4(1H,3H)-diones as dual inhibitors of HIV-1 reverse transcriptase-associated RNase H and integrase. Bioorg Med Chem27:3836-3845 (2019) [PubMed] Article Gao, P; Cheng, X; Sun, L; Song, S; Álvarez, M; Luczkowiak, J; Pannecouque, C; De Clercq, E; Menéndez-Arias, L; Zhan, P; Liu, X Design, synthesis and biological evaluation of 3-hydroxyquinazoline-2,4(1H,3H)-diones as dual inhibitors of HIV-1 reverse transcriptase-associated RNase H and integrase. Bioorg Med Chem27:3836-3845 (2019) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Reverse transcriptase/RNaseH |
|---|
| Name: | Reverse transcriptase/RNaseH |
|---|
| Synonyms: | HIV-1 Reverse Transcriptase RNase H | Human immunodeficiency virus type 1 reverse transcriptase | Reverse transcriptase/RNaseH |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 65229.15 |
|---|
| Organism: | Human immunodeficiency virus 1 |
|---|
| Description: | ChEMBL_1473730 |
|---|
| Residue: | 566 |
|---|
| Sequence: | PISPIETVPVKLKPGMDGPKVKQWPLTEEKIKALVEICTEMEKEGKISKIGPENPYNTPV
FAIKKKDSTKWRKLVDFRELNKRTQDFWEVQLGIPHPAGLKKRKSVTVLDVGDAYFSVPL
DEDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFRKQNPDIVI
YQYMDDLYVGSDLEIGQHRTKIEELRQHLLRWGLTTPDKKHQKEPPFLWMGYELHPDKWT
VQPIVLPEKDSWTVNDIQKLVGKLNWASQIYPGIRVRQLCKLLRGTKALTEVIPLTEEAE
LELAENREILKEPVHGVYYDPSKDLIAEIQKQGQGQWTYQIYQEPFKNLRTGKYARMRGA
HTNDVKQLTEAVQKITTESIVIWGKTPKFKLPIQKETWETWWTEYWQATWIPEWEFVNTP
PLVKLWYQLEKEPIVGAETFYVDGAANRETKLGKAGYVTNRGRQKVVTLTDTTNQKTELQ
AIYLALQDSGLEVNIVTDSQYALGIIQAQPDQSESELVNQIIEQLIKKEKVYLAWVPAHK
GIGGNEQVDKLVSAGIRKVLFLDGID
|
|
|
|---|
| BDBM50521979 |
|---|
| n/a |
|---|
| Name | BDBM50521979 |
|---|
| Synonyms: | CHEMBL4577620 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H10F3N3O5S |
|---|
| Mol. Mass. | 401.317 |
|---|
| SMILES | On1c(=O)[nH]c2ccc(NS(=O)(=O)c3cccc(c3)C(F)(F)F)cc2c1=O |
|---|
| Structure | 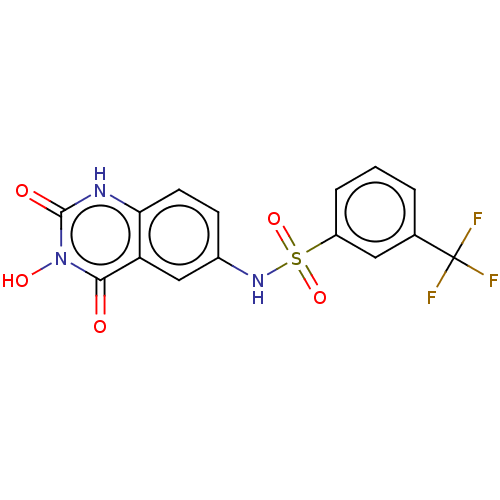
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Gao, P; Cheng, X; Sun, L; Song, S; Álvarez, M; Luczkowiak, J; Pannecouque, C; De Clercq, E; Menéndez-Arias, L; Zhan, P; Liu, X Design, synthesis and biological evaluation of 3-hydroxyquinazoline-2,4(1H,3H)-diones as dual inhibitors of HIV-1 reverse transcriptase-associated RNase H and integrase. Bioorg Med Chem27:3836-3845 (2019) [PubMed] Article
Gao, P; Cheng, X; Sun, L; Song, S; Álvarez, M; Luczkowiak, J; Pannecouque, C; De Clercq, E; Menéndez-Arias, L; Zhan, P; Liu, X Design, synthesis and biological evaluation of 3-hydroxyquinazoline-2,4(1H,3H)-diones as dual inhibitors of HIV-1 reverse transcriptase-associated RNase H and integrase. Bioorg Med Chem27:3836-3845 (2019) [PubMed] Article