

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Dual specificity protein kinase TTK | ||
| Ligand | BDBM50533123 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_1924421 (CHEMBL4427377) | ||
| IC50 | 2.8±n/a nM | ||
| Citation |  Liu, Y; Laufer, R; Patel, NK; Ng, G; Sampson, PB; Li, SW; Lang, Y; Feher, M; Brokx, R; Beletskaya, I; Hodgson, R; Plotnikova, O; Awrey, DE; Qiu, W; Chirgadze, NY; Mason, JM; Wei, X; Lin, DC; Che, Y; Kiarash, R; Fletcher, GC; Mak, TW; Bray, MR; Pauls, HW Discovery of Pyrazolo[1,5-a]pyrimidine TTK Inhibitors: CFI-402257 is a Potent, Selective, Bioavailable Anticancer Agent. ACS Med Chem Lett7:671-5 (2016) [PubMed] Article Liu, Y; Laufer, R; Patel, NK; Ng, G; Sampson, PB; Li, SW; Lang, Y; Feher, M; Brokx, R; Beletskaya, I; Hodgson, R; Plotnikova, O; Awrey, DE; Qiu, W; Chirgadze, NY; Mason, JM; Wei, X; Lin, DC; Che, Y; Kiarash, R; Fletcher, GC; Mak, TW; Bray, MR; Pauls, HW Discovery of Pyrazolo[1,5-a]pyrimidine TTK Inhibitors: CFI-402257 is a Potent, Selective, Bioavailable Anticancer Agent. ACS Med Chem Lett7:671-5 (2016) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Dual specificity protein kinase TTK | |||
| Name: | Dual specificity protein kinase TTK | ||
| Synonyms: | Dual specificity protein kinase (TTK) | Dual specificity protein kinase TTK (MPS1) | MPS1 | MPS1L1 | Monopolar Spindle 1 (MPS1) | Monopolar Spindle 1 (Mps-1) | Monopolar Spindle 1 Kinase (MPS1) | Phosphotyrosine picked threonine-protein kinase (MPS1) | TTK | TTK_HUMAN | ||
| Type: | Protein | ||
| Mol. Mass.: | 97086.79 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P33981 | ||
| Residue: | 857 | ||
| Sequence: |
| ||
| BDBM50533123 | |||
| n/a | |||
| Name | BDBM50533123 | ||
| Synonyms: | CHEMBL4540859 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C29H32N6O3 | ||
| Mol. Mass. | 512.6028 | ||
| SMILES | Cc1cc(ccc1C(=O)NC1CC1)-c1cnn2c(NC[C@H]3CC[C@H](O)CC3)cc(Oc3cccnc3)nc12 |r,wU:20.21,wD:23.25,(11.07,-27.86,;12.13,-26.74,;11.7,-25.27,;12.77,-24.16,;14.26,-24.52,;14.7,-25.99,;13.63,-27.11,;14.06,-28.59,;13,-29.7,;15.56,-28.96,;16.62,-27.84,;18.1,-27.4,;16.98,-26.34,;12.34,-22.68,;13.29,-21.47,;12.42,-20.19,;10.94,-20.63,;9.64,-19.82,;9.69,-18.28,;8.38,-17.47,;7.02,-18.2,;6.97,-19.74,;5.61,-20.46,;4.3,-19.65,;2.94,-20.37,;4.36,-18.11,;5.71,-17.39,;8.29,-20.55,;8.25,-22.09,;6.89,-22.82,;6.85,-24.36,;5.49,-25.08,;5.45,-26.62,;6.76,-27.43,;8.12,-26.69,;8.16,-25.15,;9.55,-22.89,;10.89,-22.16,)| | ||
| Structure | 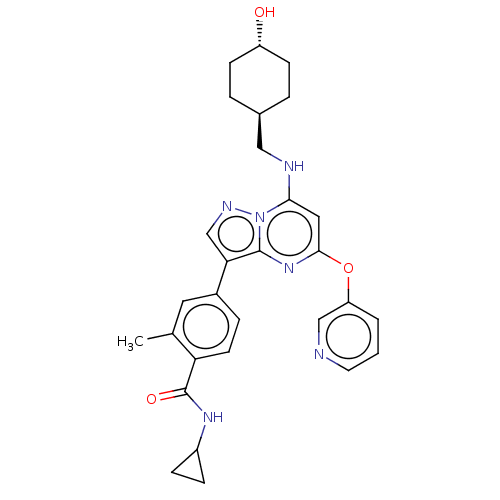
| ||