 Found 1820 hits with Last Name = 'sampson' and Initial = 'pb'
Found 1820 hits with Last Name = 'sampson' and Initial = 'pb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073585
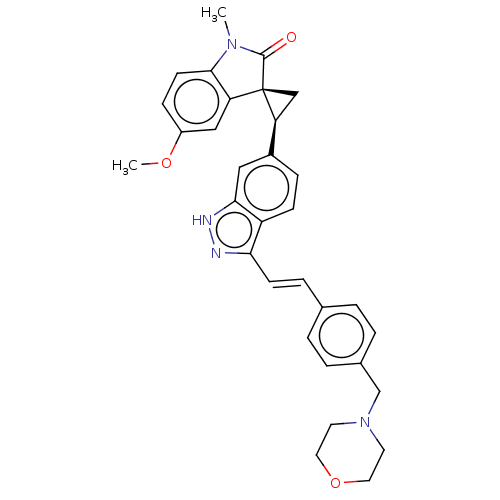
(CHEMBL3408945 | US10358436, Example A102 | US99078...)Show SMILES COc1ccc2N(C)C(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCOCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive binding to PLK4 (unknown origin) by double reciprocal plot analysis in presence of ATP |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50512456
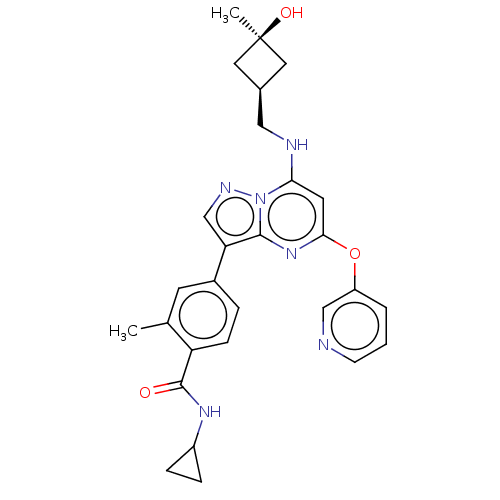
(CHEMBL4469414)Show SMILES Cc1cc(ccc1C(=O)NC1CC1)-c1cnn2c(NC[C@H]3C[C@@](C)(O)C3)cc(Oc3cccnc3)nc12 |r,wU:20.21,22.25,(48.46,-32.37,;49.53,-31.26,;49.1,-29.78,;50.17,-28.67,;51.66,-29.03,;52.09,-30.5,;51.03,-31.63,;51.46,-33.1,;50.39,-34.22,;52.95,-33.47,;54.02,-32.36,;55.49,-31.92,;54.38,-30.85,;49.74,-27.2,;50.69,-25.98,;49.82,-24.7,;48.34,-25.14,;47.04,-24.33,;47.09,-22.79,;45.78,-21.98,;44.42,-22.71,;43.98,-24.18,;42.51,-23.74,;41.01,-23.33,;41.41,-24.82,;42.95,-22.26,;45.69,-25.06,;45.64,-26.6,;44.29,-27.33,;44.25,-28.87,;42.89,-29.59,;42.85,-31.13,;44.16,-31.94,;45.52,-31.2,;45.56,-29.66,;46.95,-27.4,;48.29,-26.68,)| Show InChI InChI=1S/C28H30N6O3/c1-17-10-19(5-8-22(17)27(35)32-20-6-7-20)23-16-31-34-24(30-14-18-12-28(2,36)13-18)11-25(33-26(23)34)37-21-4-3-9-29-15-21/h3-5,8-11,15-16,18,20,30,36H,6-7,12-14H2,1-2H3,(H,32,35)/t18-,28+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant N-terminal GST-tagged and sumo-tagged human TTK (1 to 275 residues) expressed in Escherichia coli pre-incubated... |
ACS Med Chem Lett 7: 671-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00485
BindingDB Entry DOI: 10.7270/Q2K35Z46 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50512456

(CHEMBL4469414)Show SMILES Cc1cc(ccc1C(=O)NC1CC1)-c1cnn2c(NC[C@H]3C[C@@](C)(O)C3)cc(Oc3cccnc3)nc12 |r,wU:20.21,22.25,(48.46,-32.37,;49.53,-31.26,;49.1,-29.78,;50.17,-28.67,;51.66,-29.03,;52.09,-30.5,;51.03,-31.63,;51.46,-33.1,;50.39,-34.22,;52.95,-33.47,;54.02,-32.36,;55.49,-31.92,;54.38,-30.85,;49.74,-27.2,;50.69,-25.98,;49.82,-24.7,;48.34,-25.14,;47.04,-24.33,;47.09,-22.79,;45.78,-21.98,;44.42,-22.71,;43.98,-24.18,;42.51,-23.74,;41.01,-23.33,;41.41,-24.82,;42.95,-22.26,;45.69,-25.06,;45.64,-26.6,;44.29,-27.33,;44.25,-28.87,;42.89,-29.59,;42.85,-31.13,;44.16,-31.94,;45.52,-31.2,;45.56,-29.66,;46.95,-27.4,;48.29,-26.68,)| Show InChI InChI=1S/C28H30N6O3/c1-17-10-19(5-8-22(17)27(35)32-20-6-7-20)23-16-31-34-24(30-14-18-12-28(2,36)13-18)11-25(33-26(23)34)37-21-4-3-9-29-15-21/h3-5,8-11,15-16,18,20,30,36H,6-7,12-14H2,1-2H3,(H,32,35)/t18-,28+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant N-terminal GST-tagged and sumo-tagged human TTK (1 to 275 residues) expressed in Escherichia coli pre-incubated... |
ACS Med Chem Lett 7: 671-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00485
BindingDB Entry DOI: 10.7270/Q2K35Z46 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073587

(CHEMBL3408947 | US10358436, Example A185 | US20230...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive binding to PLK4 (unknown origin) by double reciprocal plot analysis in presence of ATP |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50081537
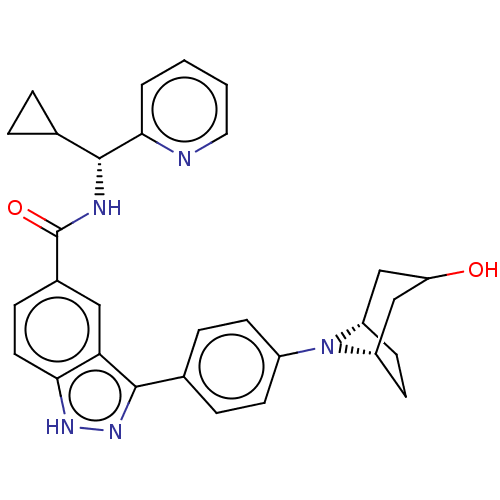
(CHEMBL3422092)Show SMILES [H][C@]12CC[C@@]([H])(CC(O)C1)N2c1ccc(cc1)-c1n[nH]c2ccc(cc12)C(=O)N[C@H](C1CC1)c1ccccn1 |r,@:7| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of amino terminal GST-fused full length human TTK using His6-SUMO-TTK-N as substrate by Lineweaver-Burk plot analysis in prese... |
J Med Chem 58: 3366-92 (2015)
Article DOI: 10.1021/jm501740a
BindingDB Entry DOI: 10.7270/Q2Q52RCN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073586
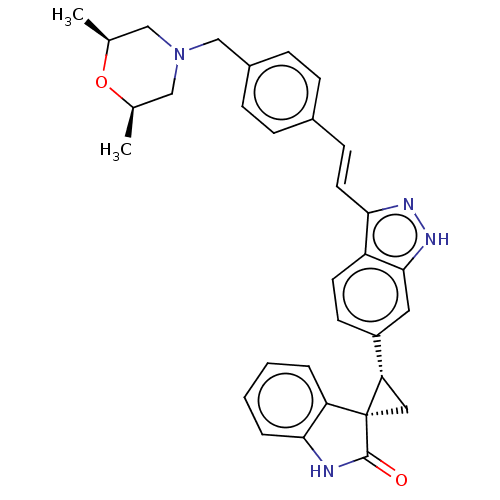
(CHEMBL3408946 | US10358436, Example A198 | US99078...)Show SMILES C[C@H]1CN(Cc2ccc(\C=C\c3n[nH]c4cc(ccc34)[C@@H]3C[C@@]33C(=O)Nc4ccccc34)cc2)C[C@@H](C)O1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive binding to PLK4 (unknown origin) by double reciprocal plot analysis in presence of ATP |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50587686
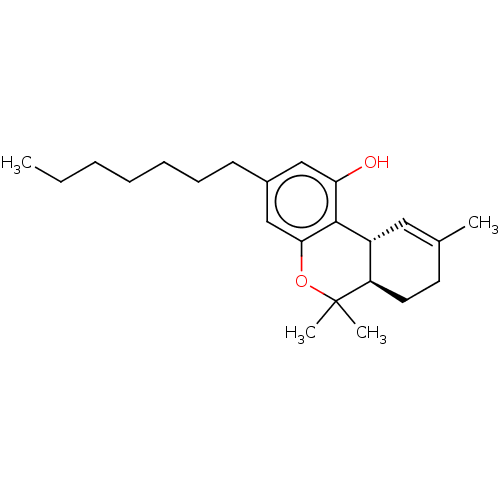
(CHEMBL5171161)Show SMILES [H][C@@]12C=C(C)CC[C@@]1([H])C(C)(C)Oc1cc(CCCCCCC)cc(O)c21 |r,t:2| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00965
BindingDB Entry DOI: 10.7270/Q2057KWP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM25117

(AG-013736 | AXITINIB | N-methyl-2-({3-[(E)-2-(pyri...)Show SMILES CNC(=O)c1ccccc1Sc1ccc2c(\C=C\c3ccccn3)n[nH]c2c1 Show InChI InChI=1S/C22H18N4OS/c1-23-22(27)18-7-2-3-8-21(18)28-16-10-11-17-19(25-26-20(17)14-16)12-9-15-6-4-5-13-24-15/h2-14H,1H3,(H,23,27)(H,25,26)/b12-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) |
J Med Chem 58: 130-46 (2015)
Article DOI: 10.1021/jm500537u
BindingDB Entry DOI: 10.7270/Q2125V9W |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50587686

(CHEMBL5171161)Show SMILES [H][C@@]12C=C(C)CC[C@@]1([H])C(C)(C)Oc1cc(CCCCCCC)cc(O)c21 |r,t:2| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00965
BindingDB Entry DOI: 10.7270/Q2057KWP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) |
J Med Chem 58: 130-46 (2015)
Article DOI: 10.1021/jm500537u
BindingDB Entry DOI: 10.7270/Q2125V9W |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50139046
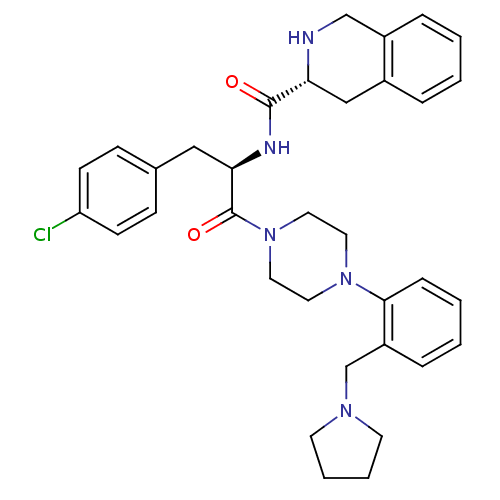
(1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...)Show SMILES Clc1ccc(C[C@@H](NC(=O)[C@H]2Cc3ccccc3CN2)C(=O)N2CCN(CC2)c2ccccc2CN2CCCC2)cc1 Show InChI InChI=1S/C34H40ClN5O2/c35-29-13-11-25(12-14-29)21-31(37-33(41)30-22-26-7-1-2-8-27(26)23-36-30)34(42)40-19-17-39(18-20-40)32-10-4-3-9-28(32)24-38-15-5-6-16-38/h1-4,7-14,30-31,36H,5-6,15-24H2,(H,37,41)/t30-,31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50139032
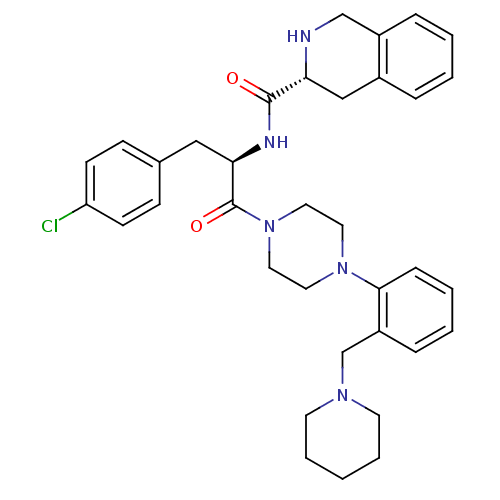
(1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...)Show SMILES Clc1ccc(C[C@@H](NC(=O)[C@H]2Cc3ccccc3CN2)C(=O)N2CCN(CC2)c2ccccc2CN2CCCCC2)cc1 Show InChI InChI=1S/C35H42ClN5O2/c36-30-14-12-26(13-15-30)22-32(38-34(42)31-23-27-8-2-3-9-28(27)24-37-31)35(43)41-20-18-40(19-21-41)33-11-5-4-10-29(33)25-39-16-6-1-7-17-39/h2-5,8-15,31-32,37H,1,6-7,16-25H2,(H,38,42)/t31-,32-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50139027
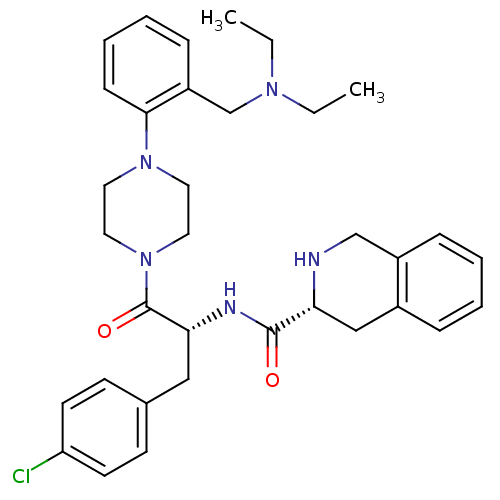
((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...)Show SMILES CCN(CC)Cc1ccccc1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C34H42ClN5O2/c1-3-38(4-2)24-28-11-7-8-12-32(28)39-17-19-40(20-18-39)34(42)31(21-25-13-15-29(35)16-14-25)37-33(41)30-22-26-9-5-6-10-27(26)23-36-30/h5-16,30-31,36H,3-4,17-24H2,1-2H3,(H,37,41)/t30-,31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50139043
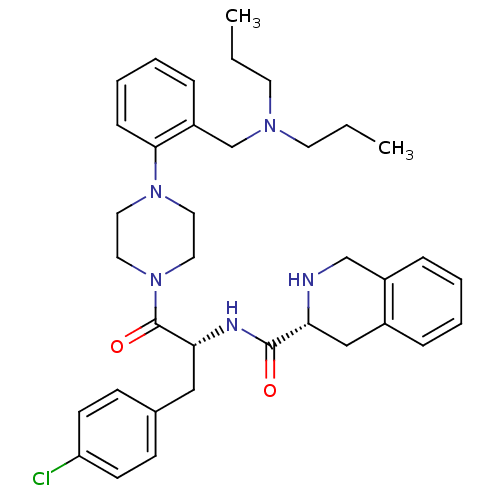
(1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...)Show SMILES CCCN(CCC)Cc1ccccc1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C36H46ClN5O2/c1-3-17-40(18-4-2)26-30-11-7-8-12-34(30)41-19-21-42(22-20-41)36(44)33(23-27-13-15-31(37)16-14-27)39-35(43)32-24-28-9-5-6-10-29(28)25-38-32/h5-16,32-33,38H,3-4,17-26H2,1-2H3,(H,39,43)/t32-,33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50139028
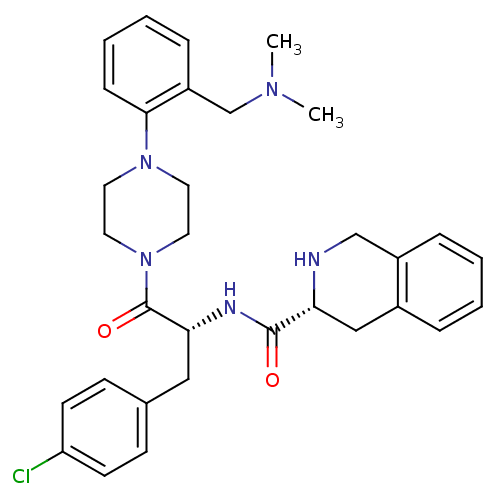
((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...)Show SMILES CN(C)Cc1ccccc1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C32H38ClN5O2/c1-36(2)22-26-9-5-6-10-30(26)37-15-17-38(18-16-37)32(40)29(19-23-11-13-27(33)14-12-23)35-31(39)28-20-24-7-3-4-8-25(24)21-34-28/h3-14,28-29,34H,15-22H2,1-2H3,(H,35,39)/t28-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50511105
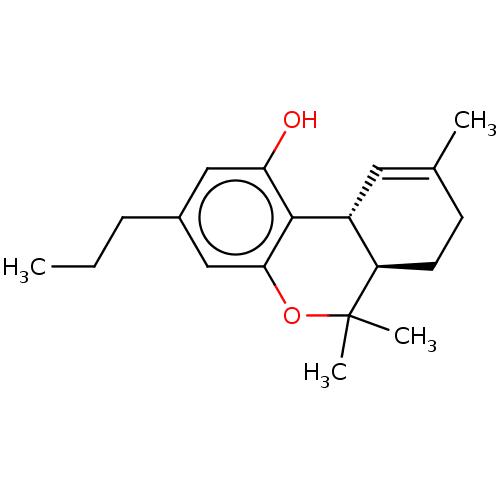
(GWP-42004 | Gwp42004 | O-4394 | THCV | Tetrahydroc...)Show SMILES [H][C@@]12C=C(C)CC[C@@]1([H])C(C)(C)Oc1cc(CCC)cc(O)c21 |r,t:2| Show InChI InChI=1S/C19H26O2/c1-5-6-13-10-16(20)18-14-9-12(2)7-8-15(14)19(3,4)21-17(18)11-13/h9-11,14-15,20H,5-8H2,1-4H3/t14-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00965
BindingDB Entry DOI: 10.7270/Q2057KWP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50061117

(6,6,9-Trimethyl-3-pentyl-6H-benzo[c]chromen-1-ol |...)Show InChI InChI=1S/C21H26O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h9-13,22H,5-8H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00965
BindingDB Entry DOI: 10.7270/Q2057KWP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50061117

(6,6,9-Trimethyl-3-pentyl-6H-benzo[c]chromen-1-ol |...)Show InChI InChI=1S/C21H26O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h9-13,22H,5-8H2,1-4H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00965
BindingDB Entry DOI: 10.7270/Q2057KWP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Mus musculus (Mouse)) | BDBM50511105

(GWP-42004 | Gwp42004 | O-4394 | THCV | Tetrahydroc...)Show SMILES [H][C@@]12C=C(C)CC[C@@]1([H])C(C)(C)Oc1cc(CCC)cc(O)c21 |r,t:2| Show InChI InChI=1S/C19H26O2/c1-5-6-13-10-16(20)18-14-9-12(2)7-8-15(14)19(3,4)21-17(18)11-13/h9-11,14-15,20H,5-8H2,1-4H3/t14-,15-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00965
BindingDB Entry DOI: 10.7270/Q2057KWP |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50139023
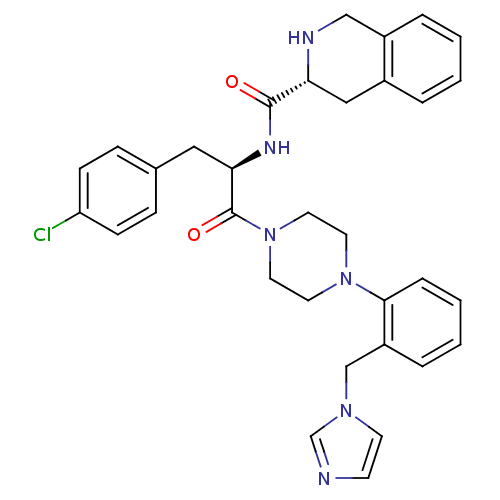
(1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...)Show SMILES Clc1ccc(C[C@@H](NC(=O)[C@H]2Cc3ccccc3CN2)C(=O)N2CCN(CC2)c2ccccc2Cn2ccnc2)cc1 Show InChI InChI=1S/C33H35ClN6O2/c34-28-11-9-24(10-12-28)19-30(37-32(41)29-20-25-5-1-2-6-26(25)21-36-29)33(42)40-17-15-39(16-18-40)31-8-4-3-7-27(31)22-38-14-13-35-23-38/h1-14,23,29-30,36H,15-22H2,(H,37,41)/t29-,30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50587686

(CHEMBL5171161)Show SMILES [H][C@@]12C=C(C)CC[C@@]1([H])C(C)(C)Oc1cc(CCCCCCC)cc(O)c21 |r,t:2| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00965
BindingDB Entry DOI: 10.7270/Q2057KWP |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50139029
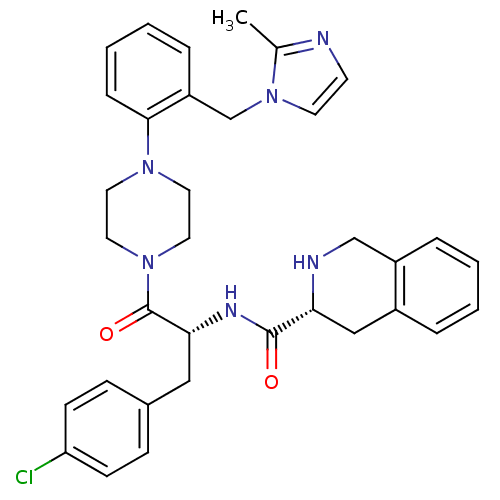
(1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...)Show SMILES Cc1nccn1Cc1ccccc1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C34H37ClN6O2/c1-24-36-14-15-41(24)23-28-8-4-5-9-32(28)39-16-18-40(19-17-39)34(43)31(20-25-10-12-29(35)13-11-25)38-33(42)30-21-26-6-2-3-7-27(26)22-37-30/h2-15,30-31,37H,16-23H2,1H3,(H,38,42)/t30-,31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50139036
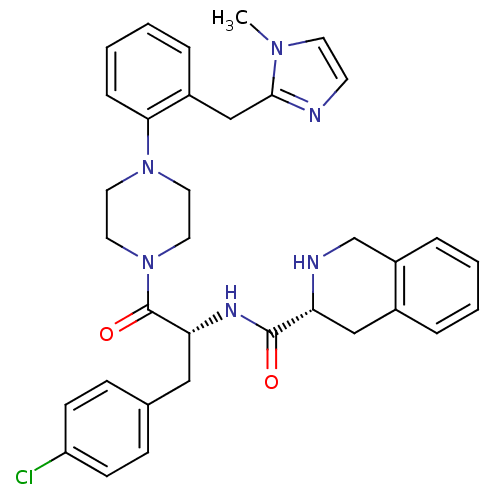
(1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...)Show SMILES Cn1ccnc1Cc1ccccc1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C34H37ClN6O2/c1-39-15-14-36-32(39)22-26-7-4-5-9-31(26)40-16-18-41(19-17-40)34(43)30(20-24-10-12-28(35)13-11-24)38-33(42)29-21-25-6-2-3-8-27(25)23-37-29/h2-15,29-30,37H,16-23H2,1H3,(H,38,42)/t29-,30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50139045
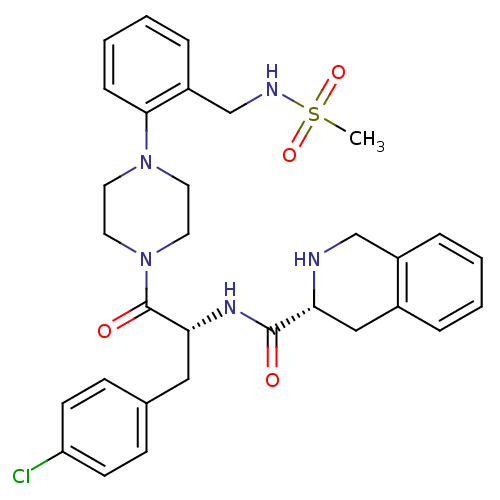
(1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...)Show SMILES CS(=O)(=O)NCc1ccccc1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C31H36ClN5O4S/c1-42(40,41)34-21-25-8-4-5-9-29(25)36-14-16-37(17-15-36)31(39)28(18-22-10-12-26(32)13-11-22)35-30(38)27-19-23-6-2-3-7-24(23)20-33-27/h2-13,27-28,33-34H,14-21H2,1H3,(H,35,38)/t27-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50134496
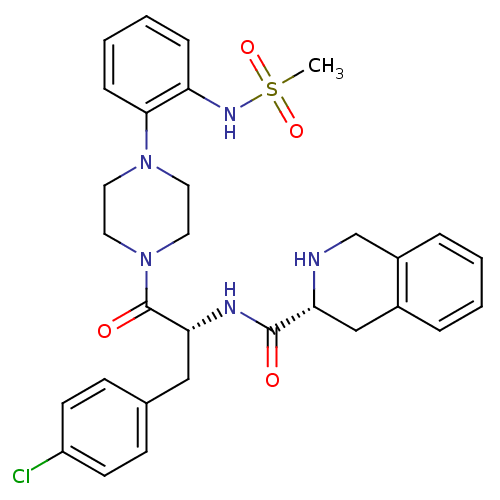
((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...)Show SMILES CS(=O)(=O)Nc1ccccc1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C30H34ClN5O4S/c1-41(39,40)34-25-8-4-5-9-28(25)35-14-16-36(17-15-35)30(38)27(18-21-10-12-24(31)13-11-21)33-29(37)26-19-22-6-2-3-7-23(22)20-32-26/h2-13,26-27,32,34H,14-20H2,1H3,(H,33,37)/t26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50139047
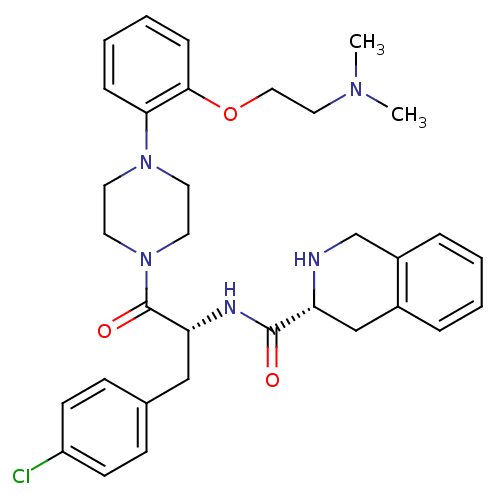
(1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...)Show SMILES CN(C)CCOc1ccccc1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C33H40ClN5O3/c1-37(2)19-20-42-31-10-6-5-9-30(31)38-15-17-39(18-16-38)33(41)29(21-24-11-13-27(34)14-12-24)36-32(40)28-22-25-7-3-4-8-26(25)23-35-28/h3-14,28-29,35H,15-23H2,1-2H3,(H,36,40)/t28-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50061117

(6,6,9-Trimethyl-3-pentyl-6H-benzo[c]chromen-1-ol |...)Show InChI InChI=1S/C21H26O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h9-13,22H,5-8H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00965
BindingDB Entry DOI: 10.7270/Q2057KWP |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50139048
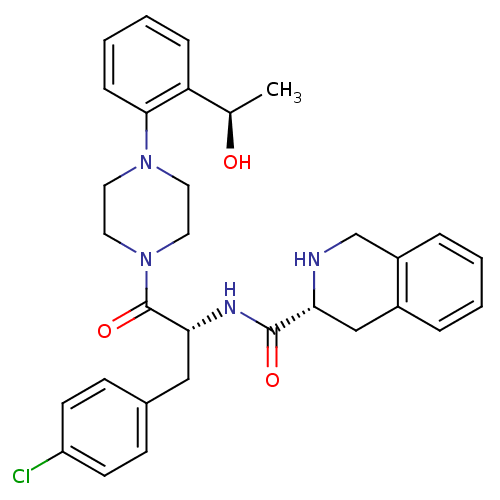
(1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...)Show SMILES C[C@@H](O)c1ccccc1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C31H35ClN4O3/c1-21(37)26-8-4-5-9-29(26)35-14-16-36(17-15-35)31(39)28(18-22-10-12-25(32)13-11-22)34-30(38)27-19-23-6-2-3-7-24(23)20-33-27/h2-13,21,27-28,33,37H,14-20H2,1H3,(H,34,38)/t21-,27-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50139044
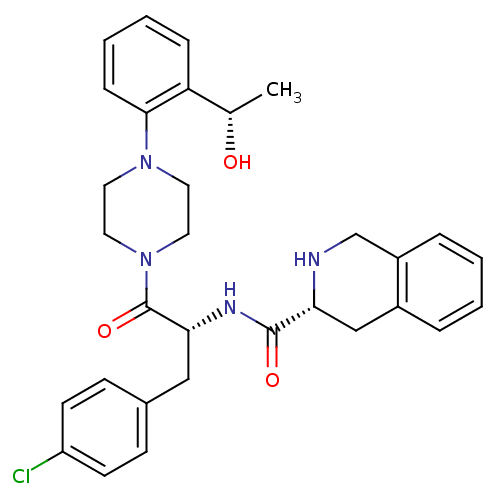
(1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...)Show SMILES C[C@H](O)c1ccccc1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C31H35ClN4O3/c1-21(37)26-8-4-5-9-29(26)35-14-16-36(17-15-35)31(39)28(18-22-10-12-25(32)13-11-22)34-30(38)27-19-23-6-2-3-7-24(23)20-33-27/h2-13,21,27-28,33,37H,14-20H2,1H3,(H,34,38)/t21-,27+,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50318487
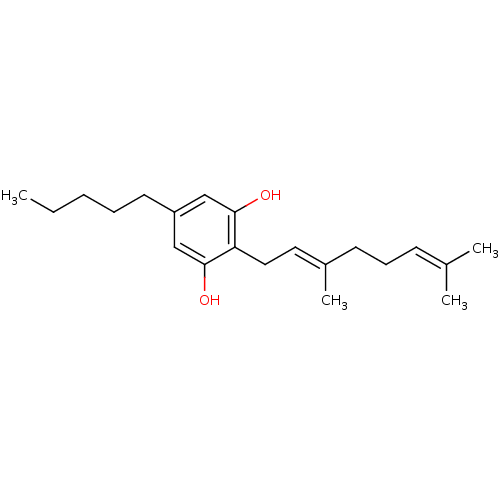
(CHEMBL497318 | Cannabigerol)Show SMILES [#6]-[#6]-[#6]-[#6]-[#6]-c1cc(-[#8])c(-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])c1 Show InChI InChI=1S/C21H32O2/c1-5-6-7-11-18-14-20(22)19(21(23)15-18)13-12-17(4)10-8-9-16(2)3/h9,12,14-15,22-23H,5-8,10-11,13H2,1-4H3/b17-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00965
BindingDB Entry DOI: 10.7270/Q2057KWP |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50139040
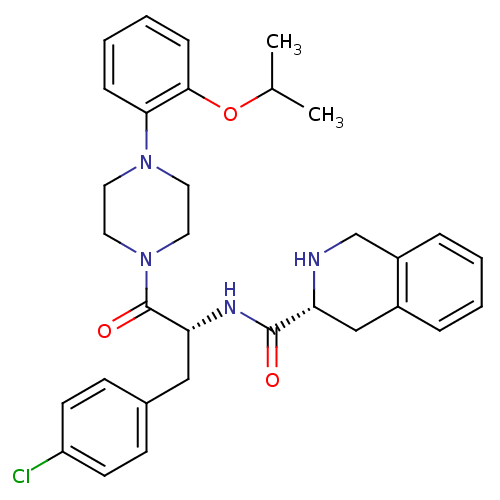
(1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C32H37ClN4O3/c1-22(2)40-30-10-6-5-9-29(30)36-15-17-37(18-16-36)32(39)28(19-23-11-13-26(33)14-12-23)35-31(38)27-20-24-7-3-4-8-25(24)21-34-27/h3-14,22,27-28,34H,15-21H2,1-2H3,(H,35,38)/t27-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50139025
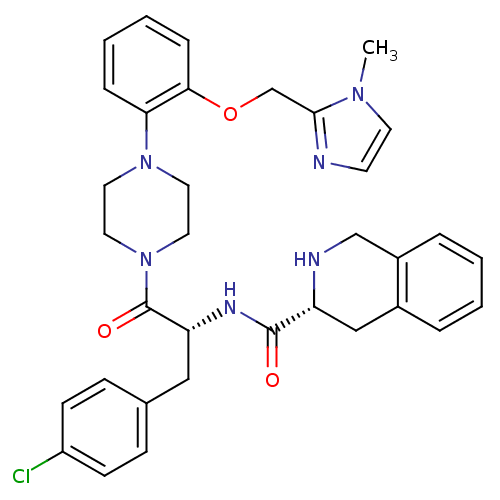
(1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...)Show SMILES Cn1ccnc1COc1ccccc1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C34H37ClN6O3/c1-39-15-14-36-32(39)23-44-31-9-5-4-8-30(31)40-16-18-41(19-17-40)34(43)29(20-24-10-12-27(35)13-11-24)38-33(42)28-21-25-6-2-3-7-26(25)22-37-28/h2-15,28-29,37H,16-23H2,1H3,(H,38,42)/t28-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50139022
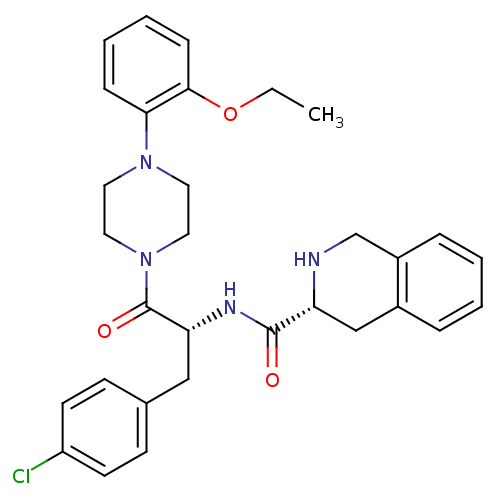
(1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...)Show SMILES CCOc1ccccc1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C31H35ClN4O3/c1-2-39-29-10-6-5-9-28(29)35-15-17-36(18-16-35)31(38)27(19-22-11-13-25(32)14-12-22)34-30(37)26-20-23-7-3-4-8-24(23)21-33-26/h3-14,26-27,33H,2,15-21H2,1H3,(H,34,37)/t26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50139038
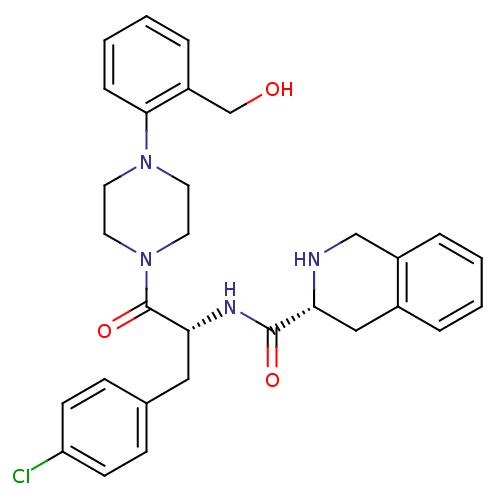
(1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...)Show SMILES OCc1ccccc1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C30H33ClN4O3/c31-25-11-9-21(10-12-25)17-27(33-29(37)26-18-22-5-1-2-6-23(22)19-32-26)30(38)35-15-13-34(14-16-35)28-8-4-3-7-24(28)20-36/h1-12,26-27,32,36H,13-20H2,(H,33,37)/t26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50139030
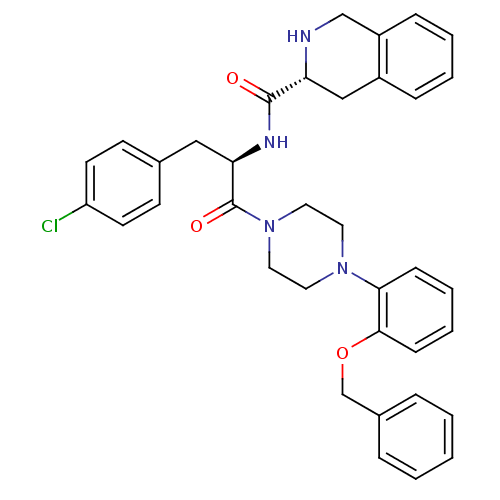
(1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...)Show SMILES Clc1ccc(C[C@@H](NC(=O)[C@H]2Cc3ccccc3CN2)C(=O)N2CCN(CC2)c2ccccc2OCc2ccccc2)cc1 Show InChI InChI=1S/C36H37ClN4O3/c37-30-16-14-26(15-17-30)22-32(39-35(42)31-23-28-10-4-5-11-29(28)24-38-31)36(43)41-20-18-40(19-21-41)33-12-6-7-13-34(33)44-25-27-8-2-1-3-9-27/h1-17,31-32,38H,18-25H2,(H,39,42)/t31-,32-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50139031
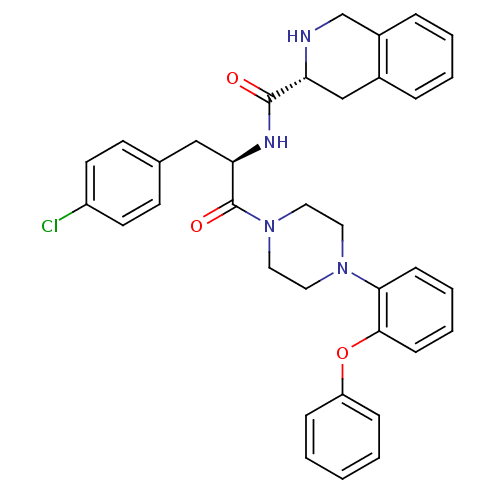
(1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...)Show SMILES Clc1ccc(C[C@@H](NC(=O)[C@H]2Cc3ccccc3CN2)C(=O)N2CCN(CC2)c2ccccc2Oc2ccccc2)cc1 Show InChI InChI=1S/C35H35ClN4O3/c36-28-16-14-25(15-17-28)22-31(38-34(41)30-23-26-8-4-5-9-27(26)24-37-30)35(42)40-20-18-39(19-21-40)32-12-6-7-13-33(32)43-29-10-2-1-3-11-29/h1-17,30-31,37H,18-24H2,(H,38,41)/t30-,31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50139023

(1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...)Show SMILES Clc1ccc(C[C@@H](NC(=O)[C@H]2Cc3ccccc3CN2)C(=O)N2CCN(CC2)c2ccccc2Cn2ccnc2)cc1 Show InChI InChI=1S/C33H35ClN6O2/c34-28-11-9-24(10-12-28)19-30(37-32(41)29-20-25-5-1-2-6-26(25)21-36-29)33(42)40-17-15-39(16-18-40)31-8-4-3-7-27(31)22-38-14-13-35-23-38/h1-14,23,29-30,36H,15-22H2,(H,37,41)/t29-,30-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human Melanocortin 5 receptor (MC5R) using [125I]NDP-alpha-MSH as a radioligand in membranes of HEK 293 cells |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
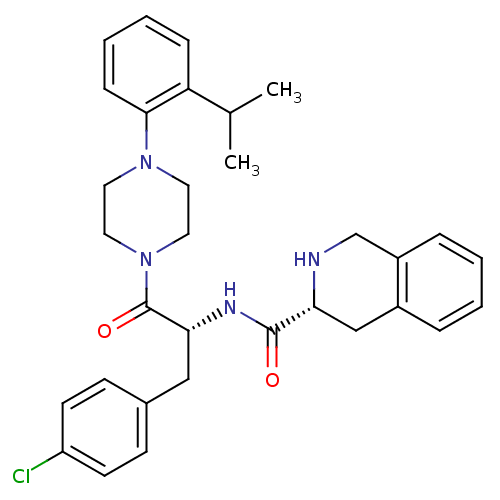
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50139039
(1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...)Show SMILES CC(C)c1ccccc1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C32H37ClN4O2/c1-22(2)27-9-5-6-10-30(27)36-15-17-37(18-16-36)32(39)29(19-23-11-13-26(33)14-12-23)35-31(38)28-20-24-7-3-4-8-25(24)21-34-28/h3-14,22,28-29,34H,15-21H2,1-2H3,(H,35,38)/t28-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50511105

(GWP-42004 | Gwp42004 | O-4394 | THCV | Tetrahydroc...)Show SMILES [H][C@@]12C=C(C)CC[C@@]1([H])C(C)(C)Oc1cc(CCC)cc(O)c21 |r,t:2| Show InChI InChI=1S/C19H26O2/c1-5-6-13-10-16(20)18-14-9-12(2)7-8-15(14)19(3,4)21-17(18)11-13/h9-11,14-15,20H,5-8H2,1-4H3/t14-,15-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00965
BindingDB Entry DOI: 10.7270/Q2057KWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50061117

(6,6,9-Trimethyl-3-pentyl-6H-benzo[c]chromen-1-ol |...)Show InChI InChI=1S/C21H26O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h9-13,22H,5-8H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00965
BindingDB Entry DOI: 10.7270/Q2057KWP |
More data for this
Ligand-Target Pair | |
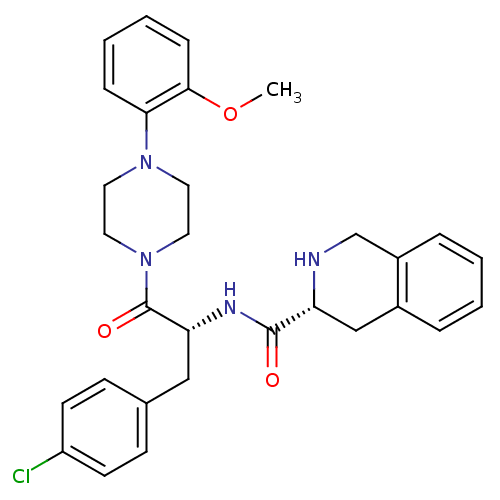
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50139041
(1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...)Show SMILES COc1ccccc1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C30H33ClN4O3/c1-38-28-9-5-4-8-27(28)34-14-16-35(17-15-34)30(37)26(18-21-10-12-24(31)13-11-21)33-29(36)25-19-22-6-2-3-7-23(22)20-32-25/h2-13,25-26,32H,14-20H2,1H3,(H,33,36)/t25-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50061117

(6,6,9-Trimethyl-3-pentyl-6H-benzo[c]chromen-1-ol |...)Show InChI InChI=1S/C21H26O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h9-13,22H,5-8H2,1-4H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00965
BindingDB Entry DOI: 10.7270/Q2057KWP |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50139042
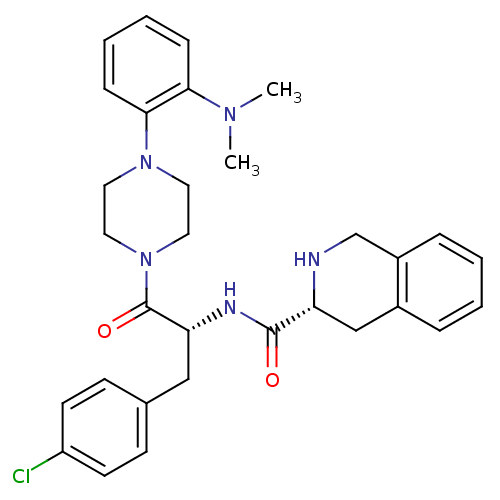
(1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...)Show SMILES CN(C)c1ccccc1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C31H36ClN5O2/c1-35(2)28-9-5-6-10-29(28)36-15-17-37(18-16-36)31(39)27(19-22-11-13-25(32)14-12-22)34-30(38)26-20-23-7-3-4-8-24(23)21-33-26/h3-14,26-27,33H,15-21H2,1-2H3,(H,34,38)/t26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50134496

((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...)Show SMILES CS(=O)(=O)Nc1ccccc1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C30H34ClN5O4S/c1-41(39,40)34-25-8-4-5-9-28(25)35-14-16-36(17-15-35)30(38)27(18-21-10-12-24(31)13-11-21)33-29(37)26-19-22-6-2-3-7-23(22)20-32-26/h2-13,26-27,32,34H,14-20H2,1H3,(H,33,37)/t26-,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human Melanocortin 5 receptor (MC5R) using [125I]NDP-alpha-MSH as a radioligand in membranes of HEK 293 cells |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50318487

(CHEMBL497318 | Cannabigerol)Show SMILES [#6]-[#6]-[#6]-[#6]-[#6]-c1cc(-[#8])c(-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])c1 Show InChI InChI=1S/C21H32O2/c1-5-6-7-11-18-14-20(22)19(21(23)15-18)13-12-17(4)10-8-9-16(2)3/h9,12,14-15,22-23H,5-8,10-11,13H2,1-4H3/b17-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00965
BindingDB Entry DOI: 10.7270/Q2057KWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
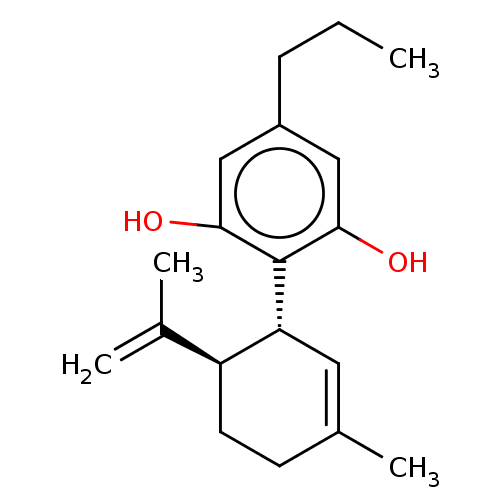
(Homo sapiens (Human)) | BDBM50532215
(CBD-V | CBDV | Cannabidivarin | GWP42006)Show SMILES [H][C@]1(CCC(C)=C[C@H]1c1c(O)cc(CCC)cc1O)C(C)=C |r,c:5| Show InChI InChI=1S/C19H26O2/c1-5-6-14-10-17(20)19(18(21)11-14)16-9-13(4)7-8-15(16)12(2)3/h9-11,15-16,20-21H,2,5-8H2,1,3-4H3/t15-,16+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00965
BindingDB Entry DOI: 10.7270/Q2057KWP |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50139034

(1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...)Show SMILES CCc1ccccc1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C31H35ClN4O2/c1-2-23-7-5-6-10-29(23)35-15-17-36(18-16-35)31(38)28(19-22-11-13-26(32)14-12-22)34-30(37)27-20-24-8-3-4-9-25(24)21-33-27/h3-14,27-28,33H,2,15-21H2,1H3,(H,34,37)/t27-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50139028

((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...)Show SMILES CN(C)Cc1ccccc1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C32H38ClN5O2/c1-36(2)22-26-9-5-6-10-30(26)37-15-17-38(18-16-37)32(40)29(19-23-11-13-27(33)14-12-23)35-31(39)28-20-24-7-3-4-8-25(24)21-34-28/h3-14,28-29,34H,15-22H2,1-2H3,(H,35,39)/t28-,29-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human Melanocortin 5 receptor (MC5R) using [125I]NDP-alpha-MSH as a radioligand in membranes of HEK 293 cells |
J Med Chem 47: 744-55 (2004)
Article DOI: 10.1021/jm0304109
BindingDB Entry DOI: 10.7270/Q2DJ5F21 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50532215

(CBD-V | CBDV | Cannabidivarin | GWP42006)Show SMILES [H][C@]1(CCC(C)=C[C@H]1c1c(O)cc(CCC)cc1O)C(C)=C |r,c:5| Show InChI InChI=1S/C19H26O2/c1-5-6-14-10-17(20)19(18(21)11-14)16-9-13(4)7-8-15(16)12(2)3/h9-11,15-16,20-21H,2,5-8H2,1,3-4H3/t15-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00965
BindingDB Entry DOI: 10.7270/Q2057KWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A1
(Homo sapiens (Human)) | BDBM50532215

(CBD-V | CBDV | Cannabidivarin | GWP42006)Show SMILES [H][C@]1(CCC(C)=C[C@H]1c1c(O)cc(CCC)cc1O)C(C)=C |r,c:5| Show InChI InChI=1S/C19H26O2/c1-5-6-14-10-17(20)19(18(21)11-14)16-9-13(4)7-8-15(16)12(2)3/h9-11,15-16,20-21H,2,5-8H2,1,3-4H3/t15-,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00965
BindingDB Entry DOI: 10.7270/Q2057KWP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data