 Found 56914 hits with Last Name = 'che' and Initial = 'y'
Found 56914 hits with Last Name = 'che' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50011320
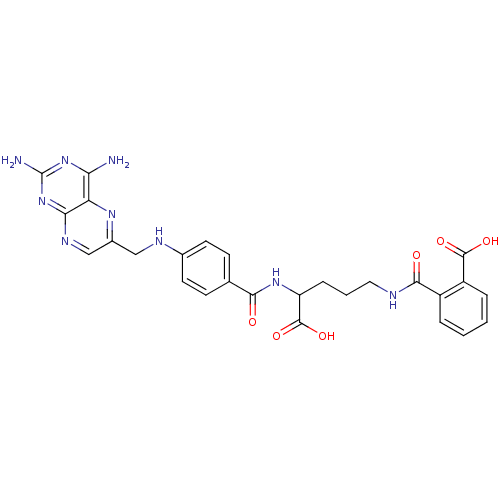
(CHEMBL18155 | N-(4-Carboxy-4-{4-[(2,4-diamino-pter...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C27H27N9O6/c28-21-20-22(36-27(29)35-21)32-13-16(33-20)12-31-15-9-7-14(8-10-15)23(37)34-19(26(41)42)6-3-11-30-24(38)17-4-1-2-5-18(17)25(39)40/h1-2,4-5,7-10,13,19,31H,3,6,11-12H2,(H,30,38)(H,34,37)(H,39,40)(H,41,42)(H4,28,29,32,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068813
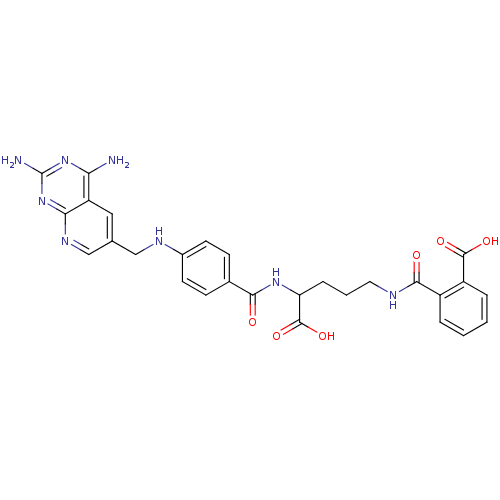
(CHEMBL149962 | N-(4-Carboxy-4-{4-[(2,4-diamino-pyr...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C28H28N8O6/c29-22-20-12-15(14-33-23(20)36-28(30)35-22)13-32-17-9-7-16(8-10-17)24(37)34-21(27(41)42)6-3-11-31-25(38)18-4-1-2-5-19(18)26(39)40/h1-2,4-5,7-10,12,14,21,32H,3,6,11,13H2,(H,31,38)(H,34,37)(H,39,40)(H,41,42)(H4,29,30,33,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068812
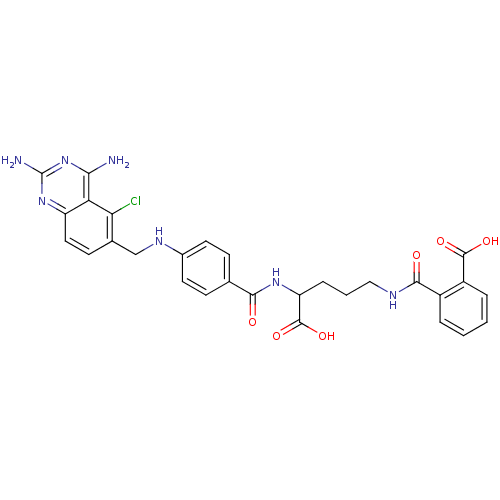
(CHEMBL146917 | N-(4-Carboxy-4-{4-[(2,4-diamino-5-c...)Show SMILES Nc1nc(N)c2c(Cl)c(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C29H28ClN7O6/c30-23-16(9-12-20-22(23)24(31)37-29(32)36-20)14-34-17-10-7-15(8-11-17)25(38)35-21(28(42)43)6-3-13-33-26(39)18-4-1-2-5-19(18)27(40)41/h1-2,4-5,7-12,21,34H,3,6,13-14H2,(H,33,39)(H,35,38)(H,40,41)(H,42,43)(H4,31,32,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068810
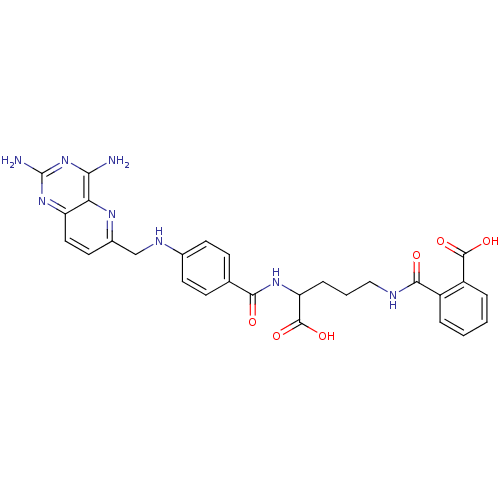
(CHEMBL149164 | N-(4-Carboxy-4-{4-[(2,4-diamino-pyr...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C28H28N8O6/c29-23-22-20(35-28(30)36-23)12-11-17(33-22)14-32-16-9-7-15(8-10-16)24(37)34-21(27(41)42)6-3-13-31-25(38)18-4-1-2-5-19(18)26(39)40/h1-2,4-5,7-12,21,32H,3,6,13-14H2,(H,31,38)(H,34,37)(H,39,40)(H,41,42)(H4,29,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068811
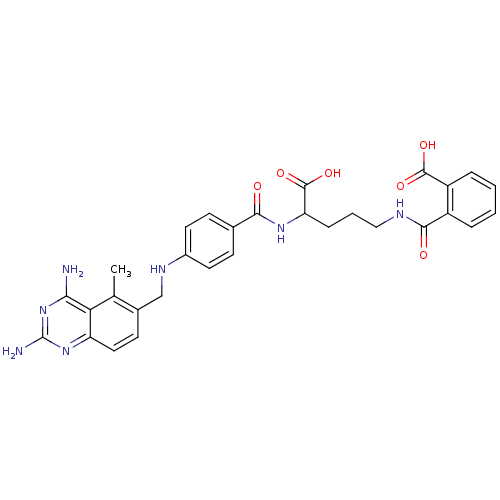
(CHEMBL149218 | N-(4-Carboxy-4-{4-[(2,4-diamino-5-m...)Show SMILES Cc1c(CNc2ccc(cc2)C(=O)NC(CCCNC(=O)c2ccccc2C(O)=O)C(O)=O)ccc2nc(N)nc(N)c12 Show InChI InChI=1S/C30H31N7O6/c1-16-18(10-13-22-24(16)25(31)37-30(32)36-22)15-34-19-11-8-17(9-12-19)26(38)35-23(29(42)43)7-4-14-33-27(39)20-5-2-3-6-21(20)28(40)41/h2-3,5-6,8-13,23,34H,4,7,14-15H2,1H3,(H,33,39)(H,35,38)(H,40,41)(H,42,43)(H4,31,32,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068808
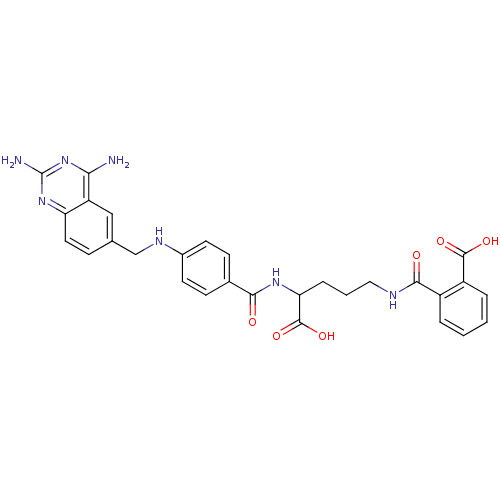
(CHEMBL297088 | N-(4-Carboxy-4-{4-[(2,4-diamino-qui...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C29H29N7O6/c30-24-21-14-16(7-12-22(21)35-29(31)36-24)15-33-18-10-8-17(9-11-18)25(37)34-23(28(41)42)6-3-13-32-26(38)19-4-1-2-5-20(19)27(39)40/h1-2,4-5,7-12,14,23,33H,3,6,13,15H2,(H,32,38)(H,34,37)(H,39,40)(H,41,42)(H4,30,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068809
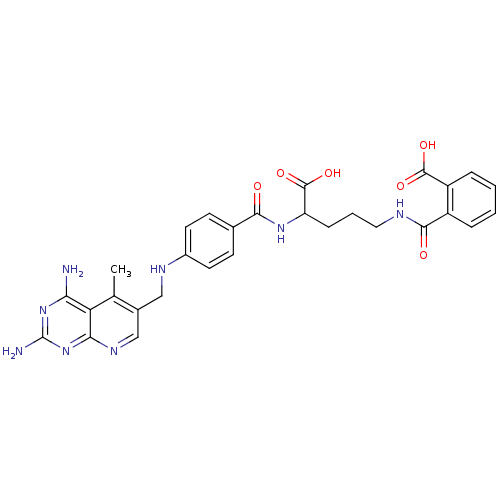
(CHEMBL150607 | N-(4-Carboxy-4-{4-[(2,4-diamino-5-m...)Show SMILES Cc1c(CNc2ccc(cc2)C(=O)NC(CCCNC(=O)c2ccccc2C(O)=O)C(O)=O)cnc2nc(N)nc(N)c12 Show InChI InChI=1S/C29H30N8O6/c1-15-17(14-34-24-22(15)23(30)36-29(31)37-24)13-33-18-10-8-16(9-11-18)25(38)35-21(28(42)43)7-4-12-32-26(39)19-5-2-3-6-20(19)27(40)41/h2-3,5-6,8-11,14,21,33H,4,7,12-13H2,1H3,(H,32,39)(H,35,38)(H,40,41)(H,42,43)(H4,30,31,34,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM106896

(US8592455, 96)Show SMILES C[C@H]1CN(C[C@@H](N)[C@@H]1O)c1ccncc1NC(=O)c1ccc(F)c(n1)-c1c(F)cccc1F |r,wU:7.8,wD:1.0,5.5,(-6,2.69,;-4.67,1.93,;-4.67,.38,;-3.33,-.38,;-2,.38,;-2,1.93,;-.67,2.69,;-3.33,2.69,;-3.33,4.23,;-3.33,-1.93,;-4.67,-2.69,;-4.67,-4.23,;-3.33,-5,;-2,-4.23,;-2,-2.69,;-.67,-1.93,;.67,-2.69,;.67,-4.23,;2,-1.93,;3.33,-2.69,;4.67,-1.93,;4.67,-.38,;6,.38,;3.33,.38,;2,-.38,;3.33,1.93,;4.67,2.69,;6,1.93,;4.67,4.23,;3.33,5,;2,4.23,;2,2.69,;.67,1.93,)| Show InChI InChI=1S/C23H22F3N5O2/c1-12-10-31(11-16(27)22(12)32)19-7-8-28-9-18(19)30-23(33)17-6-5-15(26)21(29-17)20-13(24)3-2-4-14(20)25/h2-9,12,16,22,32H,10-11,27H2,1H3,(H,30,33)/t12-,16+,22+/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PIM3 (unknown origin) |
ACS Med Chem Lett 4: 1193-7 (2013)
Article DOI: 10.1021/ml400307j
BindingDB Entry DOI: 10.7270/Q23X8840 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM106803
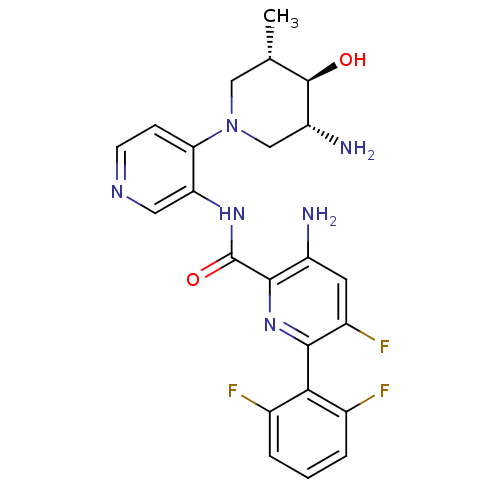
(US8592455, 3)Show SMILES C[C@H]1CN(C[C@@H](N)[C@@H]1O)c1ccncc1NC(=O)c1nc(c(F)cc1N)-c1c(F)cccc1F |r,wU:7.8,wD:1.0,5.5,(-6,2.69,;-4.67,1.93,;-4.67,.38,;-3.33,-.38,;-2,.38,;-2,1.93,;-.67,2.69,;-3.33,2.69,;-3.33,4.23,;-3.33,-1.93,;-4.67,-2.69,;-4.67,-4.23,;-3.33,-5,;-2,-4.23,;-2,-2.69,;-.67,-1.93,;.67,-2.69,;.67,-4.23,;2,-1.93,;2,-.38,;3.33,.38,;4.67,-.38,;6,.38,;4.67,-1.93,;3.33,-2.69,;3.33,-4.23,;3.33,1.93,;2,2.69,;.67,1.93,;2,4.23,;3.33,5,;4.67,4.23,;4.67,2.69,;6,1.93,)| Show InChI InChI=1S/C23H23F3N6O2/c1-11-9-32(10-16(28)22(11)33)18-5-6-29-8-17(18)30-23(34)21-15(27)7-14(26)20(31-21)19-12(24)3-2-4-13(19)25/h2-8,11,16,22,33H,9-10,27-28H2,1H3,(H,30,34)/t11-,16+,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) |
ACS Med Chem Lett 4: 1193-7 (2013)
Article DOI: 10.1021/ml400307j
BindingDB Entry DOI: 10.7270/Q23X8840 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM106896

(US8592455, 96)Show SMILES C[C@H]1CN(C[C@@H](N)[C@@H]1O)c1ccncc1NC(=O)c1ccc(F)c(n1)-c1c(F)cccc1F |r,wU:7.8,wD:1.0,5.5,(-6,2.69,;-4.67,1.93,;-4.67,.38,;-3.33,-.38,;-2,.38,;-2,1.93,;-.67,2.69,;-3.33,2.69,;-3.33,4.23,;-3.33,-1.93,;-4.67,-2.69,;-4.67,-4.23,;-3.33,-5,;-2,-4.23,;-2,-2.69,;-.67,-1.93,;.67,-2.69,;.67,-4.23,;2,-1.93,;3.33,-2.69,;4.67,-1.93,;4.67,-.38,;6,.38,;3.33,.38,;2,-.38,;3.33,1.93,;4.67,2.69,;6,1.93,;4.67,4.23,;3.33,5,;2,4.23,;2,2.69,;.67,1.93,)| Show InChI InChI=1S/C23H22F3N5O2/c1-12-10-31(11-16(27)22(12)32)19-7-8-28-9-18(19)30-23(33)17-6-5-15(26)21(29-17)20-13(24)3-2-4-14(20)25/h2-9,12,16,22,32H,10-11,27H2,1H3,(H,30,33)/t12-,16+,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) |
ACS Med Chem Lett 4: 1193-7 (2013)
Article DOI: 10.1021/ml400307j
BindingDB Entry DOI: 10.7270/Q23X8840 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM106896

(US8592455, 96)Show SMILES C[C@H]1CN(C[C@@H](N)[C@@H]1O)c1ccncc1NC(=O)c1ccc(F)c(n1)-c1c(F)cccc1F |r,wU:7.8,wD:1.0,5.5,(-6,2.69,;-4.67,1.93,;-4.67,.38,;-3.33,-.38,;-2,.38,;-2,1.93,;-.67,2.69,;-3.33,2.69,;-3.33,4.23,;-3.33,-1.93,;-4.67,-2.69,;-4.67,-4.23,;-3.33,-5,;-2,-4.23,;-2,-2.69,;-.67,-1.93,;.67,-2.69,;.67,-4.23,;2,-1.93,;3.33,-2.69,;4.67,-1.93,;4.67,-.38,;6,.38,;3.33,.38,;2,-.38,;3.33,1.93,;4.67,2.69,;6,1.93,;4.67,4.23,;3.33,5,;2,4.23,;2,2.69,;.67,1.93,)| Show InChI InChI=1S/C23H22F3N5O2/c1-12-10-31(11-16(27)22(12)32)19-7-8-28-9-18(19)30-23(33)17-6-5-15(26)21(29-17)20-13(24)3-2-4-14(20)25/h2-9,12,16,22,32H,10-11,27H2,1H3,(H,30,33)/t12-,16+,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PIM2 (unknown origin) |
ACS Med Chem Lett 4: 1193-7 (2013)
Article DOI: 10.1021/ml400307j
BindingDB Entry DOI: 10.7270/Q23X8840 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50445133
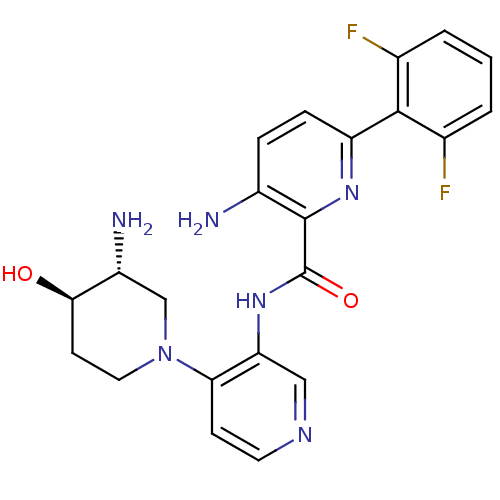
(CHEMBL3103869)Show SMILES N[C@@H]1CN(CC[C@H]1O)c1ccncc1NC(=O)c1nc(ccc1N)-c1c(F)cccc1F |r| Show InChI InChI=1S/C22H22F2N6O2/c23-12-2-1-3-13(24)20(12)16-5-4-14(25)21(28-16)22(32)29-17-10-27-8-6-18(17)30-9-7-19(31)15(26)11-30/h1-6,8,10,15,19,31H,7,9,11,25-26H2,(H,29,32)/t15-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) |
ACS Med Chem Lett 4: 1193-7 (2013)
Article DOI: 10.1021/ml400307j
BindingDB Entry DOI: 10.7270/Q23X8840 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM106803

(US8592455, 3)Show SMILES C[C@H]1CN(C[C@@H](N)[C@@H]1O)c1ccncc1NC(=O)c1nc(c(F)cc1N)-c1c(F)cccc1F |r,wU:7.8,wD:1.0,5.5,(-6,2.69,;-4.67,1.93,;-4.67,.38,;-3.33,-.38,;-2,.38,;-2,1.93,;-.67,2.69,;-3.33,2.69,;-3.33,4.23,;-3.33,-1.93,;-4.67,-2.69,;-4.67,-4.23,;-3.33,-5,;-2,-4.23,;-2,-2.69,;-.67,-1.93,;.67,-2.69,;.67,-4.23,;2,-1.93,;2,-.38,;3.33,.38,;4.67,-.38,;6,.38,;4.67,-1.93,;3.33,-2.69,;3.33,-4.23,;3.33,1.93,;2,2.69,;.67,1.93,;2,4.23,;3.33,5,;4.67,4.23,;4.67,2.69,;6,1.93,)| Show InChI InChI=1S/C23H23F3N6O2/c1-11-9-32(10-16(28)22(11)33)18-5-6-29-8-17(18)30-23(34)21-15(27)7-14(26)20(31-21)19-12(24)3-2-4-13(19)25/h2-8,11,16,22,33H,9-10,27-28H2,1H3,(H,30,34)/t11-,16+,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PIM2 (unknown origin) |
ACS Med Chem Lett 4: 1193-7 (2013)
Article DOI: 10.1021/ml400307j
BindingDB Entry DOI: 10.7270/Q23X8840 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50445124
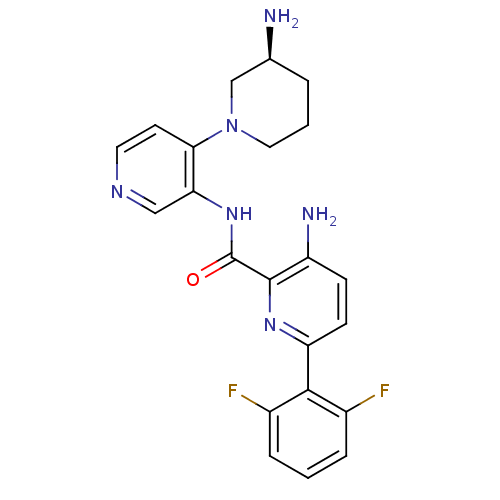
(CHEMBL3103868)Show SMILES N[C@H]1CCCN(C1)c1ccncc1NC(=O)c1nc(ccc1N)-c1c(F)cccc1F |r| Show InChI InChI=1S/C22H22F2N6O/c23-14-4-1-5-15(24)20(14)17-7-6-16(26)21(28-17)22(31)29-18-11-27-9-8-19(18)30-10-2-3-13(25)12-30/h1,4-9,11,13H,2-3,10,12,25-26H2,(H,29,31)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by AlphaScreen assay |
ACS Med Chem Lett 4: 1193-7 (2013)
Article DOI: 10.1021/ml400307j
BindingDB Entry DOI: 10.7270/Q23X8840 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM106803

(US8592455, 3)Show SMILES C[C@H]1CN(C[C@@H](N)[C@@H]1O)c1ccncc1NC(=O)c1nc(c(F)cc1N)-c1c(F)cccc1F |r,wU:7.8,wD:1.0,5.5,(-6,2.69,;-4.67,1.93,;-4.67,.38,;-3.33,-.38,;-2,.38,;-2,1.93,;-.67,2.69,;-3.33,2.69,;-3.33,4.23,;-3.33,-1.93,;-4.67,-2.69,;-4.67,-4.23,;-3.33,-5,;-2,-4.23,;-2,-2.69,;-.67,-1.93,;.67,-2.69,;.67,-4.23,;2,-1.93,;2,-.38,;3.33,.38,;4.67,-.38,;6,.38,;4.67,-1.93,;3.33,-2.69,;3.33,-4.23,;3.33,1.93,;2,2.69,;.67,1.93,;2,4.23,;3.33,5,;4.67,4.23,;4.67,2.69,;6,1.93,)| Show InChI InChI=1S/C23H23F3N6O2/c1-11-9-32(10-16(28)22(11)33)18-5-6-29-8-17(18)30-23(34)21-15(27)7-14(26)20(31-21)19-12(24)3-2-4-13(19)25/h2-8,11,16,22,33H,9-10,27-28H2,1H3,(H,30,34)/t11-,16+,22+/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PIM3 (unknown origin) |
ACS Med Chem Lett 4: 1193-7 (2013)
Article DOI: 10.1021/ml400307j
BindingDB Entry DOI: 10.7270/Q23X8840 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM50445124

(CHEMBL3103868)Show SMILES N[C@H]1CCCN(C1)c1ccncc1NC(=O)c1nc(ccc1N)-c1c(F)cccc1F |r| Show InChI InChI=1S/C22H22F2N6O/c23-14-4-1-5-15(24)20(14)17-7-6-16(26)21(28-17)22(31)29-18-11-27-9-8-19(18)30-10-2-3-13(25)12-30/h1,4-9,11,13H,2-3,10,12,25-26H2,(H,29,31)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PIM2 (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by AlphaScreen assay |
ACS Med Chem Lett 4: 1193-7 (2013)
Article DOI: 10.1021/ml400307j
BindingDB Entry DOI: 10.7270/Q23X8840 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50367055
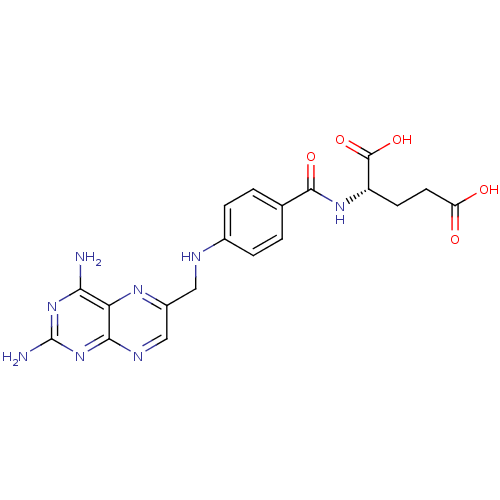
(4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cnc2n1 |r| Show InChI InChI=1S/C19H20N8O5/c20-15-14-16(27-19(21)26-15)23-8-11(24-14)7-22-10-3-1-9(2-4-10)17(30)25-12(18(31)32)5-6-13(28)29/h1-4,8,12,22H,5-7H2,(H,25,30)(H,28,29)(H,31,32)(H4,20,21,23,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098576

(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50445124

(CHEMBL3103868)Show SMILES N[C@H]1CCCN(C1)c1ccncc1NC(=O)c1nc(ccc1N)-c1c(F)cccc1F |r| Show InChI InChI=1S/C22H22F2N6O/c23-14-4-1-5-15(24)20(14)17-7-6-16(26)21(28-17)22(31)29-18-11-27-9-8-19(18)30-10-2-3-13(25)12-30/h1,4-9,11,13H,2-3,10,12,25-26H2,(H,29,31)/t13-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PIM3 (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by AlphaScreen assay |
ACS Med Chem Lett 4: 1193-7 (2013)
Article DOI: 10.1021/ml400307j
BindingDB Entry DOI: 10.7270/Q23X8840 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM106870

(US8592455, 70)Show SMILES C[C@@H]1C[C@H](N)C[C@@H](C1)c1ccncc1NC(=O)c1ccc(F)c(n1)-c1c(F)cccc1F |r,wU:6.8,3.3,1.0,(-.67,2.69,;-2,1.93,;-3.33,2.69,;-4.67,1.93,;-6,2.69,;-4.67,.38,;-3.33,-.38,;-2,.38,;-3.33,-1.93,;-4.67,-2.69,;-4.67,-4.23,;-3.33,-5,;-2,-4.23,;-2,-2.69,;-.67,-1.93,;.67,-2.69,;.67,-4.23,;2,-1.93,;3.33,-2.69,;4.67,-1.93,;4.67,-.38,;6,.38,;3.33,.38,;2,-.38,;3.33,1.93,;4.67,2.69,;6,1.93,;4.67,4.23,;3.33,5,;2,4.23,;2,2.69,;.67,1.93,)| Show InChI InChI=1S/C24H23F3N4O/c1-13-9-14(11-15(28)10-13)16-7-8-29-12-21(16)31-24(32)20-6-5-19(27)23(30-20)22-17(25)3-2-4-18(22)26/h2-8,12-15H,9-11,28H2,1H3,(H,31,32)/t13-,14+,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay |
J Med Chem 58: 8373-86 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01275
BindingDB Entry DOI: 10.7270/Q2H41VGN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM50445133

(CHEMBL3103869)Show SMILES N[C@@H]1CN(CC[C@H]1O)c1ccncc1NC(=O)c1nc(ccc1N)-c1c(F)cccc1F |r| Show InChI InChI=1S/C22H22F2N6O2/c23-12-2-1-3-13(24)20(12)16-5-4-14(25)21(28-16)22(32)29-17-10-27-8-6-18(17)30-9-7-19(31)15(26)11-30/h1-6,8,10,15,19,31H,7,9,11,25-26H2,(H,29,32)/t15-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PIM2 (unknown origin) |
ACS Med Chem Lett 4: 1193-7 (2013)
Article DOI: 10.1021/ml400307j
BindingDB Entry DOI: 10.7270/Q23X8840 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50445133

(CHEMBL3103869)Show SMILES N[C@@H]1CN(CC[C@H]1O)c1ccncc1NC(=O)c1nc(ccc1N)-c1c(F)cccc1F |r| Show InChI InChI=1S/C22H22F2N6O2/c23-12-2-1-3-13(24)20(12)16-5-4-14(25)21(28-16)22(32)29-17-10-27-8-6-18(17)30-9-7-19(31)15(26)11-30/h1-6,8,10,15,19,31H,7,9,11,25-26H2,(H,29,32)/t15-,19-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PIM3 (unknown origin) |
ACS Med Chem Lett 4: 1193-7 (2013)
Article DOI: 10.1021/ml400307j
BindingDB Entry DOI: 10.7270/Q23X8840 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM106870

(US8592455, 70)Show SMILES C[C@@H]1C[C@H](N)C[C@@H](C1)c1ccncc1NC(=O)c1ccc(F)c(n1)-c1c(F)cccc1F |r,wU:6.8,3.3,1.0,(-.67,2.69,;-2,1.93,;-3.33,2.69,;-4.67,1.93,;-6,2.69,;-4.67,.38,;-3.33,-.38,;-2,.38,;-3.33,-1.93,;-4.67,-2.69,;-4.67,-4.23,;-3.33,-5,;-2,-4.23,;-2,-2.69,;-.67,-1.93,;.67,-2.69,;.67,-4.23,;2,-1.93,;3.33,-2.69,;4.67,-1.93,;4.67,-.38,;6,.38,;3.33,.38,;2,-.38,;3.33,1.93,;4.67,2.69,;6,1.93,;4.67,4.23,;3.33,5,;2,4.23,;2,2.69,;.67,1.93,)| Show InChI InChI=1S/C24H23F3N4O/c1-13-9-14(11-15(28)10-13)16-7-8-29-12-21(16)31-24(32)20-6-5-19(27)23(30-20)22-17(25)3-2-4-18(22)26/h2-8,12-15H,9-11,28H2,1H3,(H,31,32)/t13-,14+,15-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PIM3 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay |
J Med Chem 58: 8373-86 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01275
BindingDB Entry DOI: 10.7270/Q2H41VGN |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19770
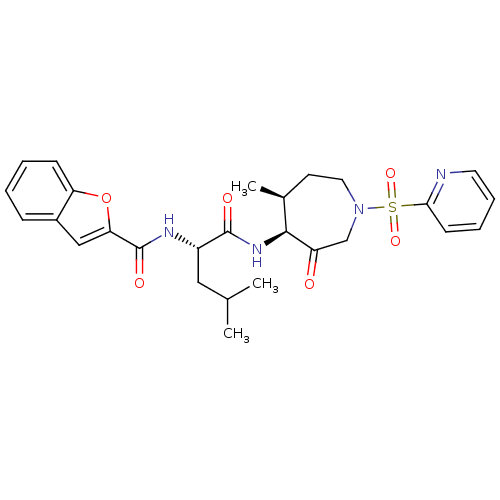
((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1[C@@H](C)CCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-20(29-27(34)23-15-19-8-4-5-9-22(19)37-23)26(33)30-25-18(3)11-13-31(16-21(25)32)38(35,36)24-10-6-7-12-28-24/h4-10,12,15,17-18,20,25H,11,13-14,16H2,1-3H3,(H,29,34)(H,30,33)/t18-,20-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00990 | -62.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM82552
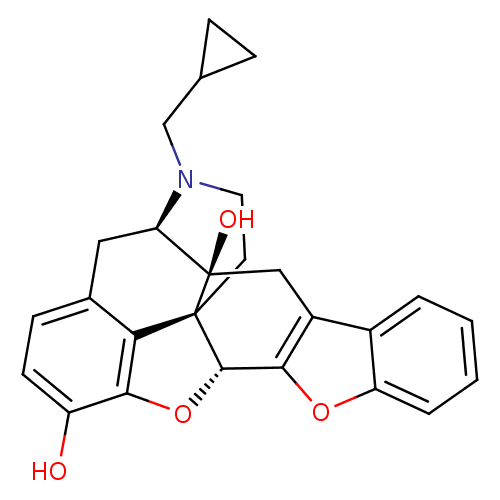
(CAS_111555-58-9 | NTB | naltrindolebenzofuran)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1oc2ccccc2c1C[C@@]35O |r| Show InChI InChI=1S/C26H25NO4/c28-18-8-7-15-11-20-26(29)12-17-16-3-1-2-4-19(16)30-22(17)24-25(26,21(15)23(18)31-24)9-10-27(20)13-14-5-6-14/h1-4,7-8,14,20,24,28-29H,5-6,9-13H2/t20-,24+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50001714
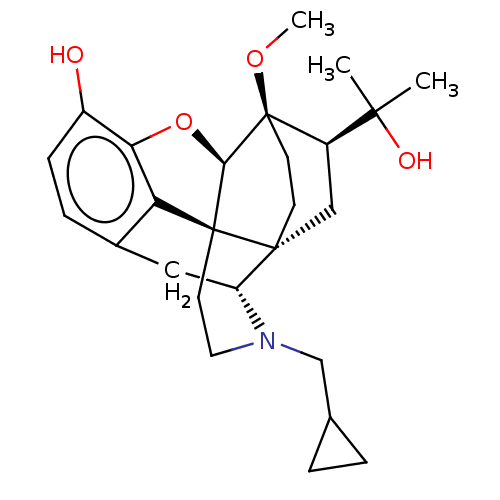
(2-[3-cyclopropylmethyl-11-hydroxy-15-methoxy-(1S,2...)Show SMILES CO[C@]12CC[C@@]3(C[C@@H]1C(C)(C)O)[C@H]1Cc4ccc(O)c5O[C@@H]2[C@]3(CCN1CC1CC1)c45 |THB:8:7:22.21:4.3| Show InChI InChI=1S/C26H35NO4/c1-23(2,29)18-13-24-8-9-26(18,30-3)22-25(24)10-11-27(14-15-4-5-15)19(24)12-16-6-7-17(28)21(31-22)20(16)25/h6-7,15,18-19,22,28-29H,4-5,8-14H2,1-3H3/t18-,19-,22-,24-,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM456924
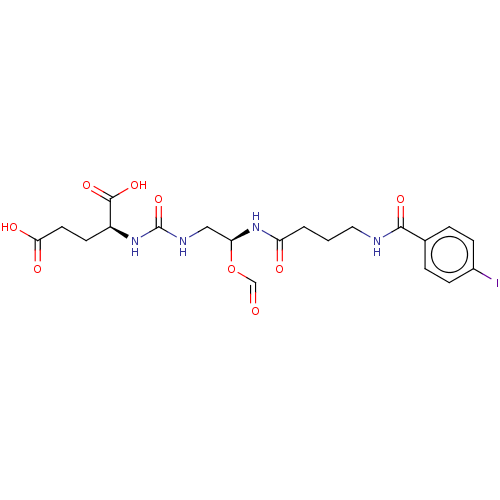
(US10736974, Compound YC-I-27)Show SMILES OC(=O)CC[C@H](NC(=O)NC[C@@H](NC(=O)CCCNC(=O)c1ccc(I)cc1)OC=O)C(O)=O |r| Show InChI InChI=1S/C20H25IN4O9/c21-13-5-3-12(4-6-13)18(30)22-9-1-2-15(27)25-16(34-11-26)10-23-20(33)24-14(19(31)32)7-8-17(28)29/h3-6,11,14,16H,1-2,7-10H2,(H,22,30)(H,25,27)(H,28,29)(H,31,32)(H2,23,24,33)/t14-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY
US Patent
| Assay Description
The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... |
US Patent US10736974 (2020)
BindingDB Entry DOI: 10.7270/Q29C71GJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50208446
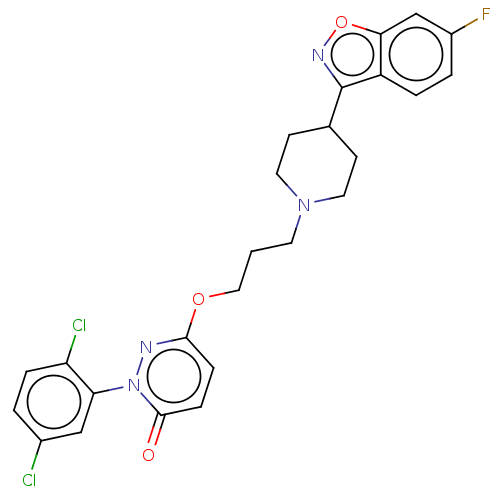
(CHEMBL3884161)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCOc2ccc(=O)n(n2)-c2cc(Cl)ccc2Cl)CC1 Show InChI InChI=1S/C25H23Cl2FN4O3/c26-17-2-5-20(27)21(14-17)32-24(33)7-6-23(29-32)34-13-1-10-31-11-8-16(9-12-31)25-19-4-3-18(28)15-22(19)35-30-25/h2-7,14-16H,1,8-13H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in Sprague-Dawley rat cerebral cortex incubated for 30 mins by liquid scintillation counting analy... |
Eur J Med Chem 124: 713-728 (2016)
Article DOI: 10.1016/j.ejmech.2016.09.008
BindingDB Entry DOI: 10.7270/Q2SQ92C5 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50246899
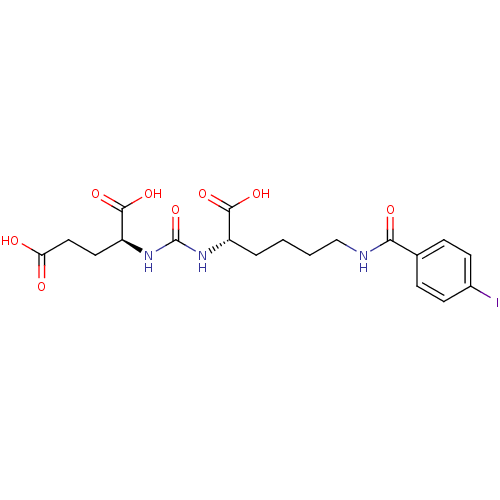
((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)c1ccc(I)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H24IN3O8/c20-12-6-4-11(5-7-12)16(26)21-10-2-1-3-13(17(27)28)22-19(31)23-14(18(29)30)8-9-15(24)25/h4-7,13-14H,1-3,8-10H2,(H,21,26)(H,24,25)(H,27,28)(H,29,30)(H2,22,23,31)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutions
Curated by ChEMBL
| Assay Description
Inhibition of PSMA in human LNCaP cell extract using [3H]NAAG by fluorescence-based assay |
J Med Chem 51: 7933-43 (2008)
Article DOI: 10.1021/jm801055h
BindingDB Entry DOI: 10.7270/Q2RV0NKJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50246899

((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)c1ccc(I)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H24IN3O8/c20-12-6-4-11(5-7-12)16(26)21-10-2-1-3-13(17(27)28)22-19(31)23-14(18(29)30)8-9-15(24)25/h4-7,13-14H,1-3,8-10H2,(H,21,26)(H,24,25)(H,27,28)(H,29,30)(H2,22,23,31)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutions
Curated by ChEMBL
| Assay Description
Inhibition of PSMA in human LNCaP cell extract using [3H]NAAG by radiometric assay |
J Med Chem 51: 7933-43 (2008)
Article DOI: 10.1021/jm801055h
BindingDB Entry DOI: 10.7270/Q2RV0NKJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50095105
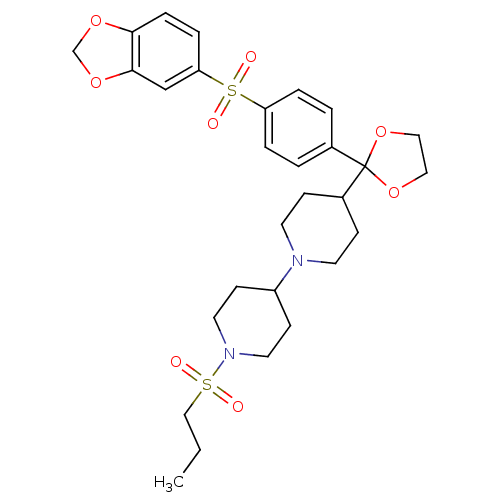
(4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1,...)Show SMILES CCCS(=O)(=O)N1CCC(CC1)N1CCC(CC1)C1(OCCO1)c1ccc(cc1)S(=O)(=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H38N2O8S2/c1-2-19-40(32,33)31-15-11-24(12-16-31)30-13-9-23(10-14-30)29(38-17-18-39-29)22-3-5-25(6-4-22)41(34,35)26-7-8-27-28(20-26)37-21-36-27/h3-8,20,23-24H,2,9-19,21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned acetylcholine receptor M2 in CHO cells. |
Bioorg Med Chem Lett 10: 2727-30 (2000)
BindingDB Entry DOI: 10.7270/Q2V40TGZ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50039022
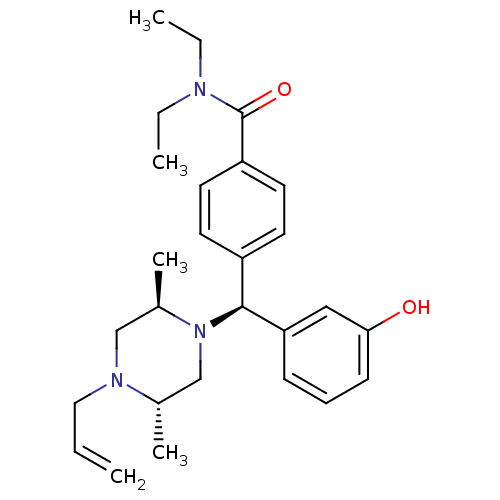
(4-[(S)-((2R,5S)-4-Allyl-2,5-dimethyl-piperazin-1-y...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@H](N1C[C@H](C)N(CC=C)C[C@H]1C)c1cccc(O)c1 Show InChI InChI=1S/C27H37N3O2/c1-6-16-29-18-21(5)30(19-20(29)4)26(24-10-9-11-25(31)17-24)22-12-14-23(15-13-22)27(32)28(7-2)8-3/h6,9-15,17,20-21,26,31H,1,7-8,16,18-19H2,2-5H3/t20-,21+,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM23334

(3-(pyridin-3-yl)propyl (2S)-1-(3,3-dimethyl-2-oxop...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)OCCCc1cccnc1 |r| Show InChI InChI=1S/C20H28N2O4/c1-4-20(2,3)17(23)18(24)22-12-6-10-16(22)19(25)26-13-7-9-15-8-5-11-21-14-15/h5,8,11,14,16H,4,6-7,9-10,12-13H2,1-3H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM320811
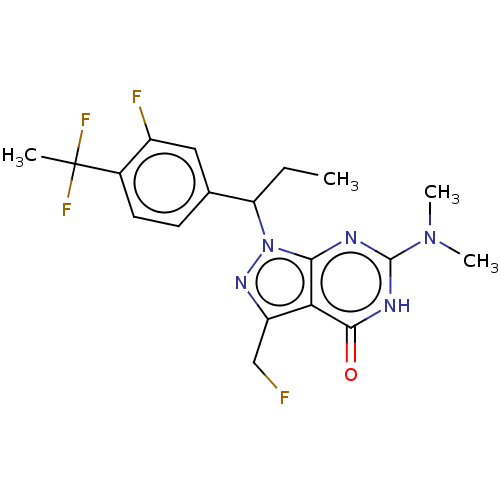
((R)- or (S)-1-(1-(4-(1,1-Difluoroethyl)-3- fluorop...)Show SMILES CCC(c1ccc(c(F)c1)C(C)(F)F)n1nc(CF)c2c1nc([nH]c2=O)N(C)C | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... |
US Patent US10174037 (2019)
BindingDB Entry DOI: 10.7270/Q29K4DB7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50387298
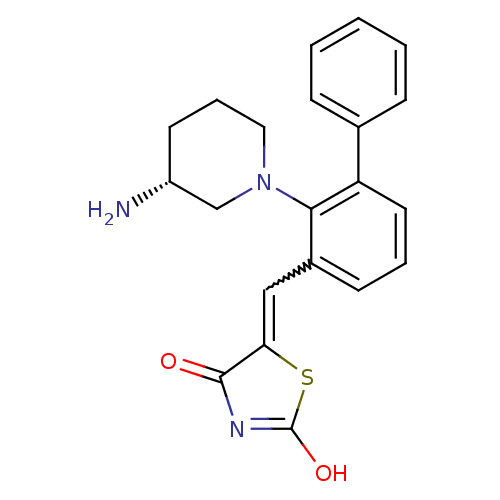
(CHEMBL2048872)Show SMILES N[C@@H]1CCCN(C1)c1c(C=C2SC(O)=NC2=O)cccc1-c1ccccc1 |r,w:9.9,c:14| Show InChI InChI=1S/C21H21N3O2S/c22-16-9-5-11-24(13-16)19-15(12-18-20(25)23-21(26)27-18)8-4-10-17(19)14-6-2-1-3-7-14/h1-4,6-8,10,12,16H,5,9,11,13,22H2,(H,23,25,26)/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay |
J Med Chem 58: 8373-86 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01275
BindingDB Entry DOI: 10.7270/Q2H41VGN |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM320710

((R)- or (S)-1-(1-(4-(1,1- Difluoroethyl)-3-fluorop...)Show SMILES CC(C)C(c1ccc(c(F)c1)C(F)(F)F)n1nc(CO)c2c1nc([nH]c2=O)N(C)C | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... |
US Patent US10174037 (2019)
BindingDB Entry DOI: 10.7270/Q29K4DB7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50387298

(CHEMBL2048872)Show SMILES N[C@@H]1CCCN(C1)c1c(C=C2SC(O)=NC2=O)cccc1-c1ccccc1 |r,w:9.9,c:14| Show InChI InChI=1S/C21H21N3O2S/c22-16-9-5-11-24(13-16)19-15(12-18-20(25)23-21(26)27-18)8-4-10-17(19)14-6-2-1-3-7-14/h1-4,6-8,10,12,16H,5,9,11,13,22H2,(H,23,25,26)/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) |
ACS Med Chem Lett 4: 1193-7 (2013)
Article DOI: 10.1021/ml400307j
BindingDB Entry DOI: 10.7270/Q23X8840 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM456928
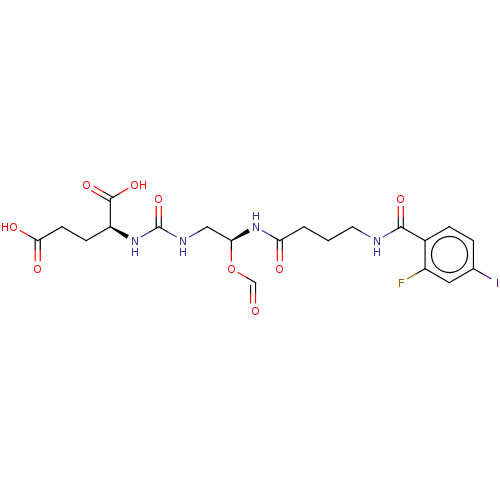
(US10736974, Compound XY-44)Show SMILES OC(=O)CC[C@H](NC(=O)NC[C@@H](NC(=O)CCCNC(=O)c1ccc(I)cc1F)OC=O)C(O)=O |r| Show InChI InChI=1S/C20H24FIN4O9/c21-13-8-11(22)3-4-12(13)18(31)23-7-1-2-15(28)26-16(35-10-27)9-24-20(34)25-14(19(32)33)5-6-17(29)30/h3-4,8,10,14,16H,1-2,5-7,9H2,(H,23,31)(H,26,28)(H,29,30)(H,32,33)(H2,24,25,34)/t14-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY
US Patent
| Assay Description
The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... |
US Patent US10736974 (2020)
BindingDB Entry DOI: 10.7270/Q29C71GJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM106870

(US8592455, 70)Show SMILES C[C@@H]1C[C@H](N)C[C@@H](C1)c1ccncc1NC(=O)c1ccc(F)c(n1)-c1c(F)cccc1F |r,wU:6.8,3.3,1.0,(-.67,2.69,;-2,1.93,;-3.33,2.69,;-4.67,1.93,;-6,2.69,;-4.67,.38,;-3.33,-.38,;-2,.38,;-3.33,-1.93,;-4.67,-2.69,;-4.67,-4.23,;-3.33,-5,;-2,-4.23,;-2,-2.69,;-.67,-1.93,;.67,-2.69,;.67,-4.23,;2,-1.93,;3.33,-2.69,;4.67,-1.93,;4.67,-.38,;6,.38,;3.33,.38,;2,-.38,;3.33,1.93,;4.67,2.69,;6,1.93,;4.67,4.23,;3.33,5,;2,4.23,;2,2.69,;.67,1.93,)| Show InChI InChI=1S/C24H23F3N4O/c1-13-9-14(11-15(28)10-13)16-7-8-29-12-21(16)31-24(32)20-6-5-19(27)23(30-20)22-17(25)3-2-4-18(22)26/h2-8,12-15H,9-11,28H2,1H3,(H,31,32)/t13-,14+,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PIM2 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay |
J Med Chem 58: 8373-86 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01275
BindingDB Entry DOI: 10.7270/Q2H41VGN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM21864
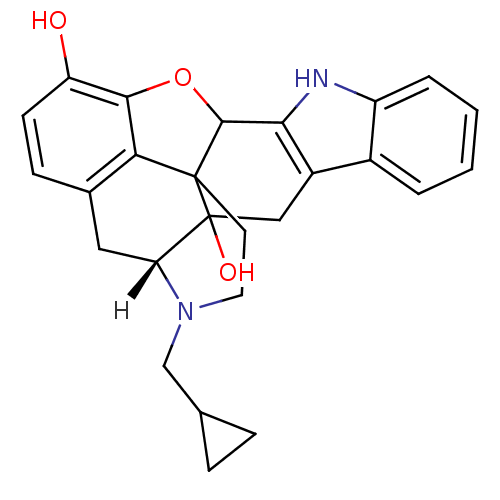
((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...)Show SMILES [H][C@@]12Cc3ccc(O)c4OC5c6[nH]c7ccccc7c6CC1(O)C5(CCN2CC1CC1)c34 |THB:27:26:21:31.2.3| Show InChI InChI=1S/C26H26N2O3/c29-19-8-7-15-11-20-26(30)12-17-16-3-1-2-4-18(16)27-22(17)24-25(26,21(15)23(19)31-24)9-10-28(20)13-14-5-6-14/h1-4,7-8,14,20,24,27,29-30H,5-6,9-13H2/t20-,24?,25?,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM82551
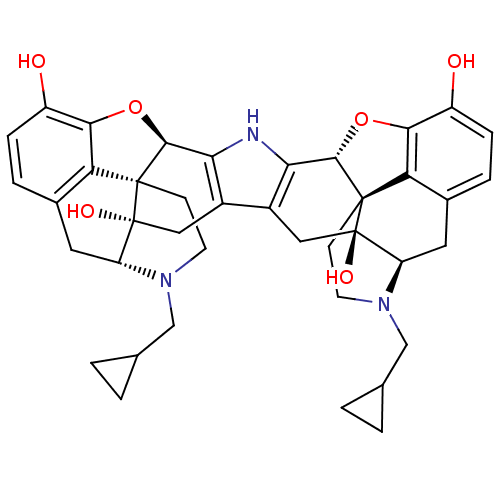
(C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2[C@@H]4Oc6c7c(C[C@H]8N(CC9CC9)CC[C@@]47[C@@]8(O)Cc2c1C[C@@]35O)ccc6O |r| Show InChI InChI=1S/C40H43N3O6/c44-25-7-5-21-13-27-39(46)15-23-24-16-40(47)28-14-22-6-8-26(45)34-30(22)38(40,10-12-43(28)18-20-3-4-20)36(49-34)32(24)41-31(23)35-37(39,29(21)33(25)48-35)9-11-42(27)17-19-1-2-19/h5-8,19-20,27-28,35-36,41,44-47H,1-4,9-18H2/t27-,28-,35+,36+,37+,38+,39-,40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM456930

(US10736974, Compound XY-59)Show SMILES OC(=O)CC[C@H](NC(=O)NC[C@@H](NC(=O)CCCNC(=O)c1ccc(Br)cc1)OC=O)C(O)=O |r| Show InChI InChI=1S/C20H25BrN4O9/c21-13-5-3-12(4-6-13)18(30)22-9-1-2-15(27)25-16(34-11-26)10-23-20(33)24-14(19(31)32)7-8-17(28)29/h3-6,11,14,16H,1-2,7-10H2,(H,22,30)(H,25,27)(H,28,29)(H,31,32)(H2,23,24,33)/t14-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY
US Patent
| Assay Description
The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... |
US Patent US10736974 (2020)
BindingDB Entry DOI: 10.7270/Q29C71GJ |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM320946

((R)- or (S)-1- (Cyclopropyl(4-(1,1- difluoroethyl)...)Show SMILES CN(C)c1nc2n(nc(CO)c2c(=O)[nH]1)C(C1CC1)c1ccc(c(F)c1)C(C)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... |
US Patent US10174037 (2019)
BindingDB Entry DOI: 10.7270/Q29K4DB7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50208470
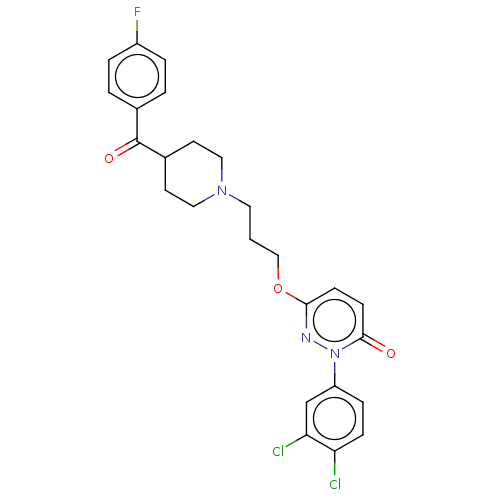
(CHEMBL3885003)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCCOc2ccc(=O)n(n2)-c2ccc(Cl)c(Cl)c2)CC1 Show InChI InChI=1S/C25H24Cl2FN3O3/c26-21-7-6-20(16-22(21)27)31-24(32)9-8-23(29-31)34-15-1-12-30-13-10-18(11-14-30)25(33)17-2-4-19(28)5-3-17/h2-9,16,18H,1,10-15H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in Sprague-Dawley rat cerebral cortex incubated for 30 mins by liquid scintillation counting analy... |
Eur J Med Chem 124: 713-728 (2016)
Article DOI: 10.1016/j.ejmech.2016.09.008
BindingDB Entry DOI: 10.7270/Q2SQ92C5 |
More data for this
Ligand-Target Pair | |
Guanylate cyclase soluble subunit alpha-1/beta-1
(Homo sapiens (Human)) | BDBM280795
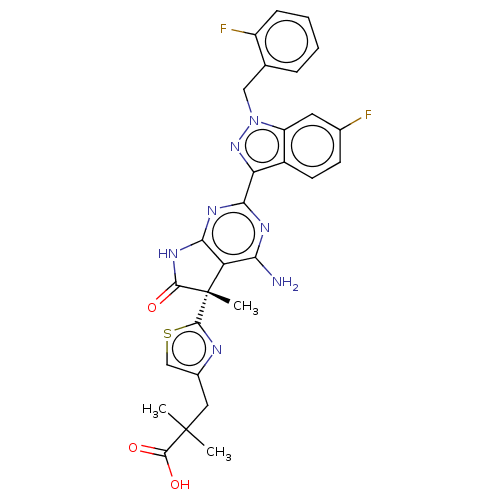
((S)-3-(2-{4-amino-2-[6- fluoro-1-(2-fluorobenzyl)-...)Show SMILES CC(C)(Cc1csc(n1)[C@]1(C)C(=O)Nc2nc(nc(N)c12)-c1nn(Cc2ccccc2F)c2cc(F)ccc12)C(O)=O |r| Show InChI InChI=1S/C29H25F2N7O3S/c1-28(2,27(40)41)11-16-13-42-26(33-16)29(3)20-22(32)34-24(35-23(20)36-25(29)39)21-17-9-8-15(30)10-19(17)38(37-21)12-14-6-4-5-7-18(14)31/h4-10,13H,11-12H2,1-3H3,(H,40,41)(H3,32,34,35,36,39)/t29-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The binding buffer was composed of 50 mM triethanolamine, pH 7.4, 3 mM MgCl2, 0.025% BSA, 2 mM dithiothreitol (DTT), 300 μM DETA/NO and 400 _... |
US Patent US10428076 (2019)
BindingDB Entry DOI: 10.7270/Q22N54P9 |
More data for this
Ligand-Target Pair | |
Guanylate cyclase soluble subunit alpha-1
(Homo sapiens (Human)) | BDBM280795

((S)-3-(2-{4-amino-2-[6- fluoro-1-(2-fluorobenzyl)-...)Show SMILES CC(C)(Cc1csc(n1)[C@]1(C)C(=O)Nc2nc(nc(N)c12)-c1nn(Cc2ccccc2F)c2cc(F)ccc12)C(O)=O |r| Show InChI InChI=1S/C29H25F2N7O3S/c1-28(2,27(40)41)11-16-13-42-26(33-16)29(3)20-22(32)34-24(35-23(20)36-25(29)39)21-17-9-8-15(30)10-19(17)38(37-21)12-14-6-4-5-7-18(14)31/h4-10,13H,11-12H2,1-3H3,(H,40,41)(H3,32,34,35,36,39)/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The binding potencies of sGC compounds to the human recombinant sGC enzyme were determined in a Size Exclusion Chromatography (SEC) competition bindi... |
US Patent US10030027 (2018)
BindingDB Entry DOI: 10.7270/Q22N549C |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM320813
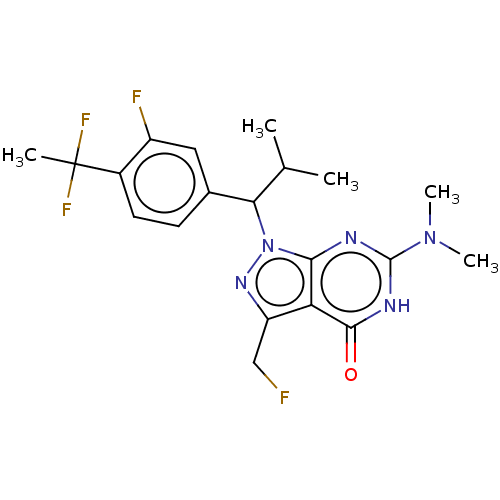
((R)- or (S)-1-(1-(4-(1,1-Difluoroethyl)-3- fluorop...)Show SMILES CC(C)C(c1ccc(c(F)c1)C(C)(F)F)n1nc(CF)c2c1nc([nH]c2=O)N(C)C | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... |
US Patent US10174037 (2019)
BindingDB Entry DOI: 10.7270/Q29K4DB7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50148931
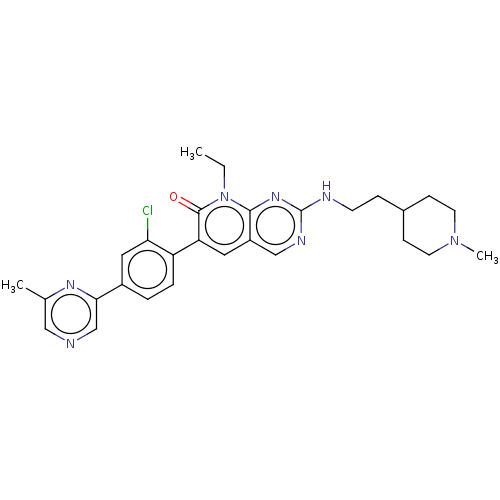
(CHEMBL3770186)Show SMILES CCn1c2nc(NCCC3CCN(C)CC3)ncc2cc(-c2ccc(cc2Cl)-c2cncc(C)n2)c1=O Show InChI InChI=1S/C28H32ClN7O/c1-4-36-26-21(16-32-28(34-26)31-10-7-19-8-11-35(3)12-9-19)13-23(27(36)37)22-6-5-20(14-24(22)29)25-17-30-15-18(2)33-25/h5-6,13-17,19H,4,7-12H2,1-3H3,(H,31,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length recombinant human N-terminal GST/His6-tagged PAK1 expressed in sf9 insect cells using tetra LRRWSLG as substrate preincubat... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112517
BindingDB Entry DOI: 10.7270/Q2Q243W7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM320712
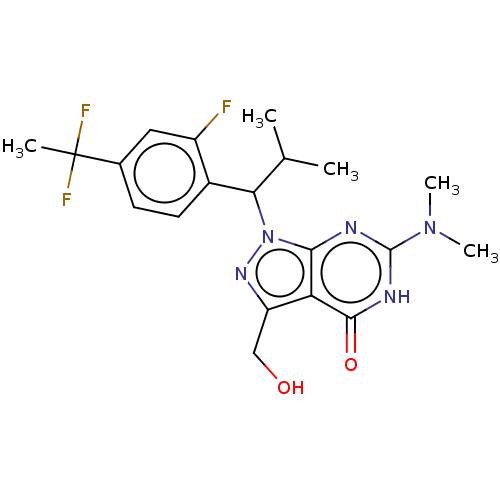
((R)- or (S)-1-(1-(4-(1,1- Difluoroethyl)-2-fluorop...)Show SMILES CC(C)C(c1ccc(cc1F)C(C)(F)F)n1nc(CO)c2c1nc([nH]c2=O)N(C)C | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... |
US Patent US10174037 (2019)
BindingDB Entry DOI: 10.7270/Q29K4DB7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data