| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Reverse transcriptase/RNaseH |
|---|
| Ligand | BDBM50533398 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1925210 (CHEMBL4428282) |
|---|
| IC50 | 1000±n/a nM |
|---|
| Citation |  Tang, J; Liu, F; Nagy, E; Miller, L; Kirby, KA; Wilson, DJ; Wu, B; Sarafianos, SG; Parniak, MA; Wang, Z 3-Hydroxypyrimidine-2,4-diones as Selective Active Site Inhibitors of HIV Reverse Transcriptase-Associated RNase H: Design, Synthesis, and Biochemical Evaluations. J Med Chem59:2648-59 (2016) [PubMed] Article Tang, J; Liu, F; Nagy, E; Miller, L; Kirby, KA; Wilson, DJ; Wu, B; Sarafianos, SG; Parniak, MA; Wang, Z 3-Hydroxypyrimidine-2,4-diones as Selective Active Site Inhibitors of HIV Reverse Transcriptase-Associated RNase H: Design, Synthesis, and Biochemical Evaluations. J Med Chem59:2648-59 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Reverse transcriptase/RNaseH |
|---|
| Name: | Reverse transcriptase/RNaseH |
|---|
| Synonyms: | HIV-1 Reverse Transcriptase RNase H | Human immunodeficiency virus type 1 reverse transcriptase | Reverse transcriptase/RNaseH |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 65229.15 |
|---|
| Organism: | Human immunodeficiency virus 1 |
|---|
| Description: | ChEMBL_1473730 |
|---|
| Residue: | 566 |
|---|
| Sequence: | PISPIETVPVKLKPGMDGPKVKQWPLTEEKIKALVEICTEMEKEGKISKIGPENPYNTPV
FAIKKKDSTKWRKLVDFRELNKRTQDFWEVQLGIPHPAGLKKRKSVTVLDVGDAYFSVPL
DEDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFRKQNPDIVI
YQYMDDLYVGSDLEIGQHRTKIEELRQHLLRWGLTTPDKKHQKEPPFLWMGYELHPDKWT
VQPIVLPEKDSWTVNDIQKLVGKLNWASQIYPGIRVRQLCKLLRGTKALTEVIPLTEEAE
LELAENREILKEPVHGVYYDPSKDLIAEIQKQGQGQWTYQIYQEPFKNLRTGKYARMRGA
HTNDVKQLTEAVQKITTESIVIWGKTPKFKLPIQKETWETWWTEYWQATWIPEWEFVNTP
PLVKLWYQLEKEPIVGAETFYVDGAANRETKLGKAGYVTNRGRQKVVTLTDTTNQKTELQ
AIYLALQDSGLEVNIVTDSQYALGIIQAQPDQSESELVNQIIEQLIKKEKVYLAWVPAHK
GIGGNEQVDKLVSAGIRKVLFLDGID
|
|
|
|---|
| BDBM50533398 |
|---|
| n/a |
|---|
| Name | BDBM50533398 |
|---|
| Synonyms: | CHEMBL4438951 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H14N4O3 |
|---|
| Mol. Mass. | 334.3288 |
|---|
| SMILES | Cn1c(Nc2cccc(c2)-c2ccc(cc2)C#N)cc(=O)n(O)c1=O |
|---|
| Structure | 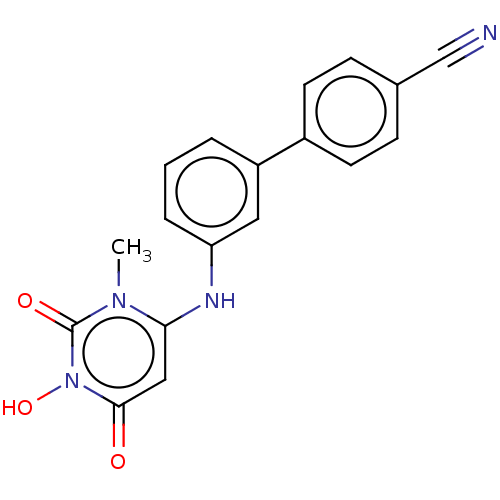
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Tang, J; Liu, F; Nagy, E; Miller, L; Kirby, KA; Wilson, DJ; Wu, B; Sarafianos, SG; Parniak, MA; Wang, Z 3-Hydroxypyrimidine-2,4-diones as Selective Active Site Inhibitors of HIV Reverse Transcriptase-Associated RNase H: Design, Synthesis, and Biochemical Evaluations. J Med Chem59:2648-59 (2016) [PubMed] Article
Tang, J; Liu, F; Nagy, E; Miller, L; Kirby, KA; Wilson, DJ; Wu, B; Sarafianos, SG; Parniak, MA; Wang, Z 3-Hydroxypyrimidine-2,4-diones as Selective Active Site Inhibitors of HIV Reverse Transcriptase-Associated RNase H: Design, Synthesis, and Biochemical Evaluations. J Med Chem59:2648-59 (2016) [PubMed] Article