| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 1A2 |
|---|
| Ligand | BDBM50598923 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2228509 (CHEMBL5142022) |
|---|
| IC50 | >10000±n/a nM |
|---|
| Citation |  Cisar, JS; Pietsch, C; DeRatt, LG; Jacoby, E; Kazmi, F; Keohane, C; Legenski, K; Matico, R; Shaffer, P; Simonnet, Y; Tanner, A; Wang, CY; Wang, W; Attar, R; Edwards, JP; Kuduk, SD -Heterocyclic 3-Pyridyl Carboxamide Inhibitors of DHODH for the Treatment of Acute Myelogenous Leukemia. J Med Chem65:11241-11256 (2022) [PubMed] Article Cisar, JS; Pietsch, C; DeRatt, LG; Jacoby, E; Kazmi, F; Keohane, C; Legenski, K; Matico, R; Shaffer, P; Simonnet, Y; Tanner, A; Wang, CY; Wang, W; Attar, R; Edwards, JP; Kuduk, SD -Heterocyclic 3-Pyridyl Carboxamide Inhibitors of DHODH for the Treatment of Acute Myelogenous Leukemia. J Med Chem65:11241-11256 (2022) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 1A2 |
|---|
| Name: | Cytochrome P450 1A2 |
|---|
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58423.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05177 |
|---|
| Residue: | 516 |
|---|
| Sequence: | MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKN
PHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDG
QSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELM
AGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFP
ILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGN
LIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLS
DRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPEL
WEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLE
FSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
|
|
|
|---|
| BDBM50598923 |
|---|
| n/a |
|---|
| Name | BDBM50598923 |
|---|
| Synonyms: | CHEMBL5193821 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H21ClF4N6O5 |
|---|
| Mol. Mass. | 548.875 |
|---|
| SMILES | CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)cnc(OC)c2Cl)c1=O |r| |
|---|
| Structure | 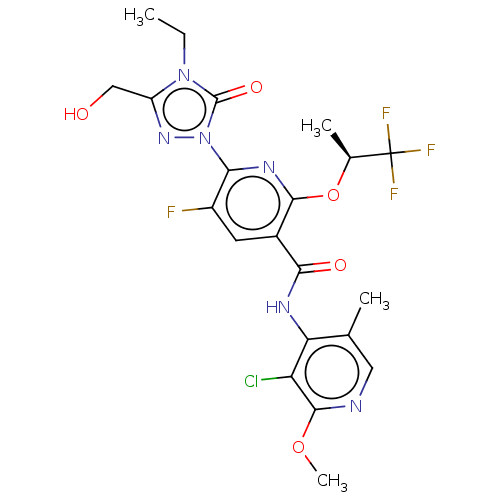
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Cisar, JS; Pietsch, C; DeRatt, LG; Jacoby, E; Kazmi, F; Keohane, C; Legenski, K; Matico, R; Shaffer, P; Simonnet, Y; Tanner, A; Wang, CY; Wang, W; Attar, R; Edwards, JP; Kuduk, SD -Heterocyclic 3-Pyridyl Carboxamide Inhibitors of DHODH for the Treatment of Acute Myelogenous Leukemia. J Med Chem65:11241-11256 (2022) [PubMed] Article
Cisar, JS; Pietsch, C; DeRatt, LG; Jacoby, E; Kazmi, F; Keohane, C; Legenski, K; Matico, R; Shaffer, P; Simonnet, Y; Tanner, A; Wang, CY; Wang, W; Attar, R; Edwards, JP; Kuduk, SD -Heterocyclic 3-Pyridyl Carboxamide Inhibitors of DHODH for the Treatment of Acute Myelogenous Leukemia. J Med Chem65:11241-11256 (2022) [PubMed] Article