| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Dual specificity mitogen-activated protein kinase kinase 1 |
|---|
| Ligand | BDBM50187822 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_383093 (CHEMBL867341) |
|---|
| IC50 | 1200±n/a nM |
|---|
| Citation |  Varaprasad, CV; Barawkar, D; El Abdellaoui, H; Chakravarty, S; Allan, M; Chen, H; Zhang, W; Wu, JZ; Tam, R; Hamatake, R; Lang, S; Hong, Z Discovery of 3-hydroxy-4-carboxyalkylamidino-5-arylamino-isothiazoles as potent MEK1 inhibitors. Bioorg Med Chem Lett16:3975-80 (2006) [PubMed] Article Varaprasad, CV; Barawkar, D; El Abdellaoui, H; Chakravarty, S; Allan, M; Chen, H; Zhang, W; Wu, JZ; Tam, R; Hamatake, R; Lang, S; Hong, Z Discovery of 3-hydroxy-4-carboxyalkylamidino-5-arylamino-isothiazoles as potent MEK1 inhibitors. Bioorg Med Chem Lett16:3975-80 (2006) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Dual specificity mitogen-activated protein kinase kinase 1 |
|---|
| Name: | Dual specificity mitogen-activated protein kinase kinase 1 |
|---|
| Synonyms: | Dual specificity mitogen-activated protein kinase (MEK) | Dual specificity mitogen-activated protein kinase kinase 1 (MEK) | Dual specificity mitogen-activated protein kinase kinase 1 (MEK1) | Dual specificity mitogen-activated protein kinase kinase 1/Mitogen-activated protein kinase 1/RAF proto-oncogene serine/threonine-protein kinase | Dual specificity mitogen-activated protein kinase kinase MEK1/2 | ERK activator kinase 1 | MAP kinase kinase 1 | MAP2K1 | MAPK/ERK kinase 1 | MAPK/ERK kinase 1 (MEK1) | MEK-1 | MEK1 | MP2K1_HUMAN | Mitogen-activated protein kinase 1 (MEK1) | PRKMK1 | VHL-MAP2K1/MAP2K2 |
|---|
| Type: | Other Protein Type |
|---|
| Mol. Mass.: | 43439.03 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Full-length human MEK-1 was generated by PCR and purified as a fusion protein from Escherichia coli lysates. |
|---|
| Residue: | 393 |
|---|
| Sequence: | MPKKKPTPIQLNPAPDGSAVNGTSSAETNLEALQKKLEELELDEQQRKRLEAFLTQKQKV
GELKDDDFEKISELGAGNGGVVFKVSHKPSGLVMARKLIHLEIKPAIRNQIIRELQVLHE
CNSPYIVGFYGAFYSDGEISICMEHMDGGSLDQVLKKAGRIPEQILGKVSIAVIKGLTYL
REKHKIMHRDVKPSNILVNSRGEIKLCDFGVSGQLIDSMANSFVGTRSYMSPERLQGTHY
SVQSDIWSMGLSLVEMAVGRYPIPPPDAKELELMFGCQVEGDAAETPPRPRTPGRPLSSY
GMDSRPPMAIFELLDYIVNEPPPKLPSGVFSLEFQDFVNKCLIKNPAERADLKQLMVHAF
IKRSDAEEVDFAGWLCSTIGLNQPSTPTHAAGV
|
|
|
|---|
| BDBM50187822 |
|---|
| n/a |
|---|
| Name | BDBM50187822 |
|---|
| Synonyms: | 5-(p-toluidino)-N-(cyclohexylmethyl)-3-hydroxyisothiazole-4-carboxamidine | CHEMBL212480 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H24N4OS |
|---|
| Mol. Mass. | 344.474 |
|---|
| SMILES | Cc1ccc(Nc2s[nH]c(=O)c2C(=N)NCC2CCCCC2)cc1 |
|---|
| Structure | 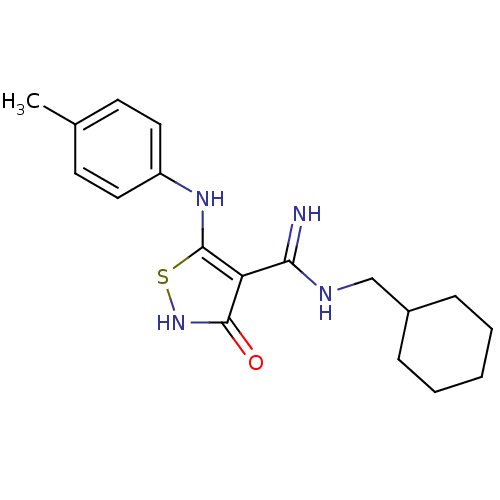
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Varaprasad, CV; Barawkar, D; El Abdellaoui, H; Chakravarty, S; Allan, M; Chen, H; Zhang, W; Wu, JZ; Tam, R; Hamatake, R; Lang, S; Hong, Z Discovery of 3-hydroxy-4-carboxyalkylamidino-5-arylamino-isothiazoles as potent MEK1 inhibitors. Bioorg Med Chem Lett16:3975-80 (2006) [PubMed] Article
Varaprasad, CV; Barawkar, D; El Abdellaoui, H; Chakravarty, S; Allan, M; Chen, H; Zhang, W; Wu, JZ; Tam, R; Hamatake, R; Lang, S; Hong, Z Discovery of 3-hydroxy-4-carboxyalkylamidino-5-arylamino-isothiazoles as potent MEK1 inhibitors. Bioorg Med Chem Lett16:3975-80 (2006) [PubMed] Article